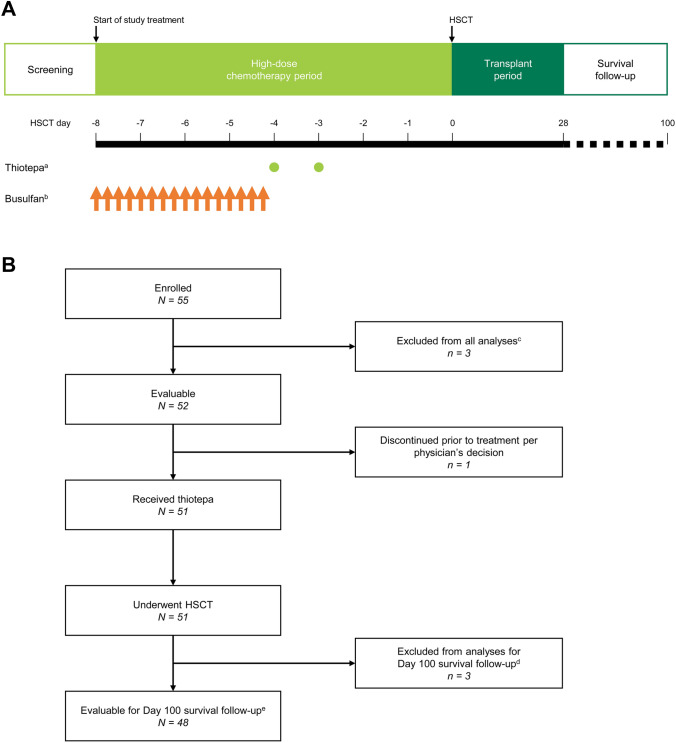
Fig. 1.
A Study design in patients with malignant lymphoma. B Patient disposition. The screening period included collection of informed consent and study enrollment. Day 0 was the day of HSCT. aThiotepa 200 mg/m2/day IV over 2 h on days -4 and -3. bBusulfan 0.8 mg/kg IV over 2 h every 6 h on days -8, -7, -6 and -5. cThree patents did not have validated data; the sponsor was unable to visit one of the study sites to verify the documentation for most of the data due to the institute’s COVID-19 precautions within the study period. Therefore, these patients were excluded from all analyses. dThree additional patients did not have validated data for survival at day 100 after HSCT due to the COVID-19 precautions at the same institute. These patients were excluded from the analysis of survival at day 100 after HSCT. Validated data for other measures, including adverse events, were available and these three patients were included in the safety analysis set. eIncludes two patients who did not complete the full 100 days (censored) due to termination of the study when thiotepa received regulatory approval for malignant lymphoma. HDT high-dose chemotherapy, HSCT hematopoietic stem cell transplantation, IV intravenously