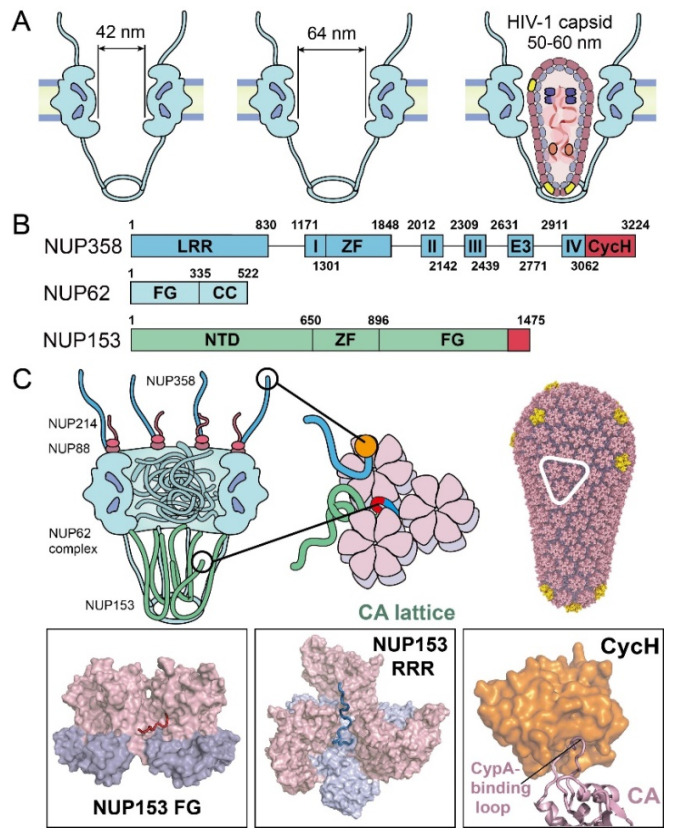
Figure 3.
NPC components known to affect nuclear import of the HIV-1 core. (A) Schematics of the diameters of the NPC central channel and the size of HIV-1 capsid. (B) Domain organization of the major capsid-interacting NUPs, including NUP358, NUP153, and NUP62. Known CA-binding fragments are colored in red. Domain labels are as follows: LRR—leucine-rich region; roman numerals I–IV—Ran binding domains I–IV; ZF—zinc finger; E3—E3 ligase domain; CycH—cyclophilin homology domain; FG—phenylalanine-glycine repeats domain; CC—coiled-coil domain; NTD—N-terminal domain. (C) Summary of virus–nucleoporin interactions. The boxed insets show structural details of the interactions. The last FG-motif at NUP153 C-terminal region occupies the FG-binding pocket, formed at the NTD–CTD interface between adjacent CA monomers (PDB 4U0C). NUP153 “RRR” motif at its C-terminus binds at the CA tri-hexamer interface [57]. NUP358 CycH binds to the CypA-binding loop on CA (PDB 4LQW).