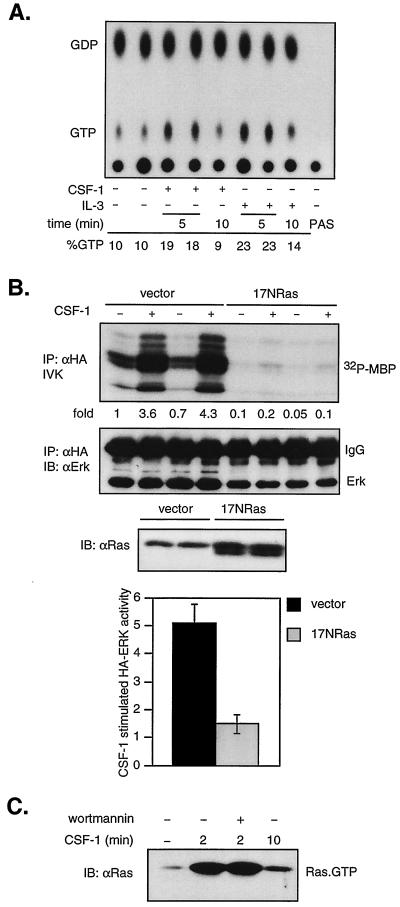
FIG. 3.
CSF-1 activation of ERK is Ras dependent, and PI3-kinase inhibition does not affect Ras activation. (A) In vivo Ras activation. 32D-CSF-1R cells were starved in medium containing reduced serum and WEHI, labeled with [32P]orthophosphate, and stimulated with CSF-1 or IL-3 for the indicated times. Ras was immunoprecipitated, and bound nucleotides were eluted and analyzed by thin-layer chromatography as described in Materials and Methods. PAS refers to a sample where lysates were bound to PAS in the absence of primary antibodies. Percent GTP is calculated as 100 × cpm (GTP)/[1.5 × cpm (GDP) + cpm (GTP)]. (B) Effect of dominant-negative Ras on CSF-1 activation of ERK. 32D-CSF-1R cells were electroporated with either 7 μg of pcDNA and 2 μg of HA-ERK or 5 μg of 17NRas and 4 μg of HA-ERK. Transfections were carried out in duplicate on the same clone as indicated. The amount of HA-ERK plasmid DNA was selected to yield approximately equivalent expression levels. (Top) HA-ERK was immunoprecipitated (IP) with anti-HA antibodies and sub- jected to an IVK assay. (Middle) The amount of immunoprecipitated HA-ERK was determined by immunoblotting (IB) with an ERK monoclonal antibody. (Lower) The expression of 17NRas was assessed by immunoblotting with a pan-Ras antibody. (Bottom) Summary of results from five independent, matched transfections with either vector or 17NRas. The graph depicts the average fold increase in HA-ERK activity induced by CSF-1 over untreated cells. The difference in CSF-1-stimulated HA-ERK activity between vector- or 17NRas-transfected cells is statistically significant (P < 0.001, two-sided Student's t test). (C) Effect of wortmannin on Ras activation. Cells were starved, pretreated or not with 200 nM wortmannin, and then stimulated with CSF-1 for the indicated times. The amount of active Ras (Ras-GTP) in total cell detergent lysates was determined in a GST-RBD pull-down assay (see Materials and Methods). Active Ras was detected by immunoblotting with a pan-Ras antibody.