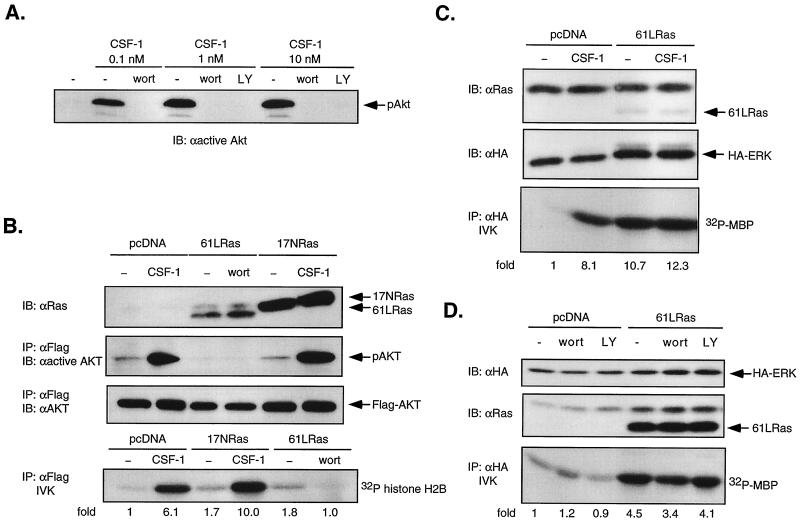
FIG. 4.
Dominant-negative Ras does not block CSF-1-activated Akt/PKB, and oncogenic Ras does not stimulate Akt activity in 32D-CSF-1R cells. (A) Effect of PI3-kinase inhibition on Akt phosphorylation. 32D-CSF-1R cells were pretreated or not with either wortmannin (wort; 200 nM) or LY294002 (LY; 30 μM) for 20 min before stimulation with 0.1, 1, or 10 nM CSF-1. Total cell lysates were analyzed for Akt phosphorylation by immunoblotting (IB) with anti-active Akt antibodies. (B) Akt activity in cells transfected with oncogenic or dominant-negative Ras. Cells were transfected with 5 μg of WT Flag-Akt and 10 μg of either vector, 61LRas, or 17NRas. Transfected Ras was verified by immunoblotting with anti-Ras antibodies. Lysates were immunoprecipitated (IP) with anti-Flag antibodies and either immunoblotted with an antibody that recognizes phosphorylated Akt (anti-active Akt) or subjected to an IVK assay with histone H2B as substrate. The amount of immunoprecipitated Akt was determined by immunoblotting with anti-Akt antibodies. (C) Oncogenic Ras activation of ERK. Cells were transfected with either 7 μg of pcDNA and 2 μg of HA-ERK or 6 μg of 61LRas, 2 μg of pcDNA, and 1 μg of HA-ERK. Ras and HA-ERK expression was detected by immunoblotting with anti-Ras and anti-HA antibodies, respectively. Lysates were analyzed for MBP kinase activity in anti-HA immunoprecipitates. (D) Effect of PI3-kinase inhibition on ERK activity stimulated by oncogenic Ras. Cells were transfected with either 4 μg of pcDNA and 4 μg of HA-ERK or 7 μg of 61LRas and 1 μg HA-ERK, starved, and left untreated or else treated with either 200 nM wortmannin or 50 μM LY294002 prior to lysis. MBP kinase activity was determined in anti-HA immunoprecipitates.