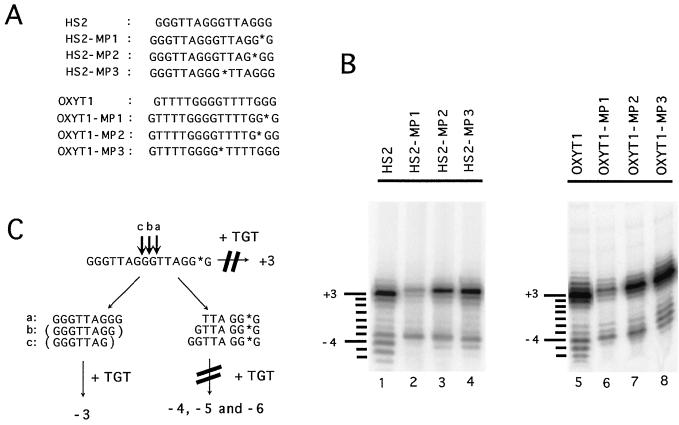
FIG. 6.
Effects of methylphosphonate linkages in the DNA primer on cleavage and extension by yeast telomerase. (A) The regular and derivatized oligonucleotides used for the reactions in panel B are listed. The location of the methylphosphonate linkage is denoted by an asterisk. (B) Polymerization assays were carried out using 0.5 μg of the various primers as indicated at the top and either 5 μg (lanes 1 to 4) or 3 μg (lanes 5 to 8) of the DEAE fraction. The lengths of the labeled products relative to the starting primers are indicated by lines and numbers to the left of the panels. (C) A schematic illustration of the cleavage-elongation pathways that can account for the reaction products visualized in lanes 1 and 2 of panel B. The nuclease is proposed to act endonucleolytically and to act predominantly in the middle G tract. As described in the text, yeast telomerase strongly prefers to extend primers that have three Gs at their 3′ end and extends these primers predominantly by 3 nt. Thus, reaction a generates two fragments that can both be efficiently extended, leading to the synthesis of the primer-3 and primer-6 products. Reactions b and c each generate only one efficient substrate, leading to the synthesis of the primer-5 and primer-4 products. The methylphosphonate substitution in MP-1 (marked by an asterisk) strongly inhibits extension of the nearby 3′ OH group by telomerase, causing the loss of the “+3” product as well as the “−4,” “−5,” and “−6” products.