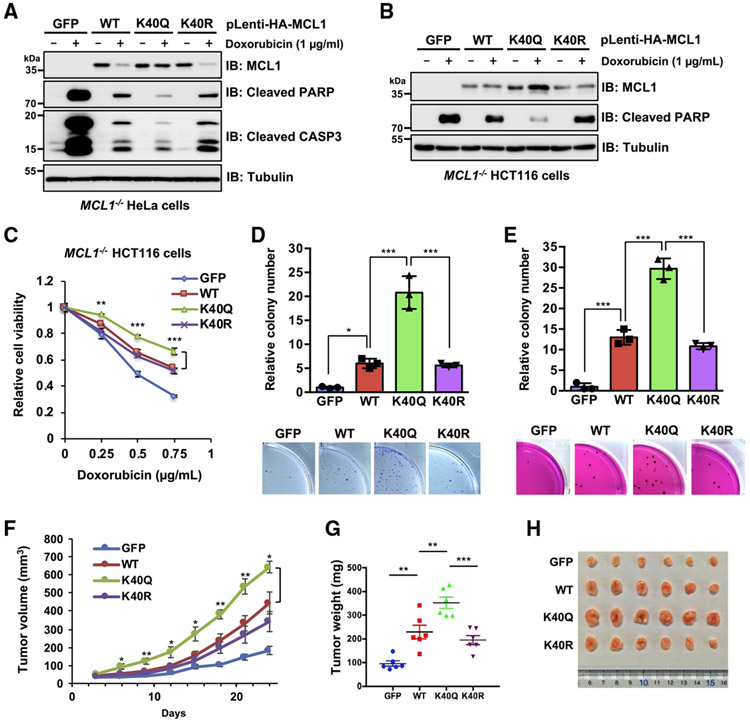
Figure 3. Acetylation-mimetic MCL1 K40Q displays enhanced anti-apoptotic function and oncogenicity.
(A and B) Ectopic expression of the acetylation-mimetic MCL1 K40Q mutant confers resistance to doxorubicin-induced downregulation. Shown is IB analysis of WCLs derived from HeLa cells (A) and HCT116 cells (B), which stably express WT MCL1, K40Q, or K40R at a level comparable to that where endogenous MCL1 is eliminated by the CRISPR-Cas9 system. The resulting cells were treated with doxorubicin (1 μg/mL) for 24 h before harvesting.
(C) Acetylation-mimetic MCL1 K40Q enhances its anti-apoptotic function. The HCT116 cells presented in (B) were treated with the indicated concentrations of doxorubicin for 24 h and then subjected to cell viability assays. Data are presented as mean ± SD; n = 3 biological replicates; **p < 0.01, ***p < 0.001.
(D and E) Acetylation-mimetic MCL1 K40Q enhances the tumorigenic activity of MCL1. A colony formation assay was conducted using HeLa cells
(D) presented in (A) and a soft agar assay using HCT116 cells (E) presented in (B). These cells were pretreated overnight with doxorubicin (0.02 μ g/mL) before plating for the assays. Data are presented as mean ± SD; n = 3 biological replicates; *p < 0.05, ***p < 0.001.
(F–H) Acetylation-mimetic MCL1 K40Q promotes tumor growth in the mouse xenograft model. HeLa cells presented in (A) were injected subcutaneously into nude mice (n = 6 for each group). Tumor growth was monitored over the indicated periods (F) and the weight of the dissected tumors (G) and images of the dissected tumors (H) are presented. Data are presented as mean ± SEM; n = 6; *p < 0.05, **p < 0.01, ***p < 0.001.
Data in (A) and (B) are representative of at least two independent experiments. See also Figure S3.