Abstract
Background: Regenerative endodontics aims to restore normal pulp function in necrotic and infected teeth, restoring protective functions, such as innate pulp immunity, pulp repair through mineralization, and pulp sensibility. The aim of this systematic review was to assess the dentin regeneration efficacy of direct pulp capping (DPC) biomaterials. Methods: The literature published between 2005 and 2021 was searched by using PubMed, Web of Science, Science Direct, Google Scholar, and Scopus databases. Clinical controlled trials, randomized controlled trials, and animal studies investigating DPC outcomes or comparing different capping materials after pulp exposure were included in this systematic review. Three independent authors performed the searches, and information was extracted by using a structured data format. Results: A total of forty studies (21 from humans and 19 from animals) were included in this systemic review. Histological examinations showed complete/partial/incomplete dentin bridge/reparative dentin formation during the pulp healing process at different follow-up periods, using different capping materials. Conclusions: Mineral trioxide aggregate (MTA) and Biodentine can induce dentin regeneration when applied over exposed pulp. This systematic review can conclude that MTA and its variants have better efficacy in the DPC procedure for dentin regeneration.
Keywords: direct pulp capping, dentin regeneration, dentin-bridge formation, reparative dentin, calcium hydroxide, mineral trioxide aggregate
1. Introduction
Vital dental pulp exposure may be caused by caries removal (caries exposure), cavity preparation where pinpoint exposure to the dental pulp (mechanical exposure), and accidental coronal pulp injury (traumatic exposure). The preservation of pulp vitality is important in all of these situations for tooth viability, nutrition, innervation, and immune defense. Dentin acts as a protective barrier that protects the tooth pulp from direct contact with potentially tissue-damaging external stimuli [1]. The formation of tertiary dentin in response to various noxious stimuli can increase the thickness of the dentin barrier [2]. The odontoblasts, which are the cells responsible for dentinogenesis, are found on the periphery of the dental pulp. These odontoblast cells could be destroyed due to severe external stimuli, such as deep dental caries. Consequently, the recruitment of progenitors and the induction of differentiation by odontoblast can take place, leading to the formation of newly generative cells, which are known as odontoblast-like cells [1].
In regenerative dentistry, DPC is a treatment procedure that utilizes the regenerative abilities of human dental pulp cells that has been described previously [3]. Pulp capping aims to facilitate the healing of injured pulp by using bioactive materials to ensure the formation of mineralized tissue or dentin bridge [4]. The use of this method may be a more conservative alternative to root-canal treatment in cases where the pulp has been exposed due to reversible injury or does not exhibit symptoms of inflammation [5]. Numerous studies were conducted to assess the effectiveness of the DPC materials with the following outcomes: pulp vitality, dentin-bridge formation, inflammation, and presence of bacteria [6,7,8]. Above all, histological analysis remains the reference standard for determining the status of the pulp and dentin-bridge formation [9]. Recently, calcium silicate–based cement has been considered as the most suitable material for pulp capping as a surface-active hard-tissue substitute because of its excellent bioactivity and biocompatibility [3]. They are widely utilized for conservative treatment, such as direct/indirect pulp capping, apexification, apexogenesis, and the repair of furcation, due to their biocompatibility, chemical bonding with tooth structure, easy handling characteristics, and good sealing ability [10].
In terms of clinical outcomes and hard-tissue formation, materials used for DPC in the exposed pulp have been evaluated [11,12,13,14]. Calcium hydroxide (CH), which has been the gold standard of care for these procedures for a long time, has antibacterial properties, and promotes healing and repair; however, it has poor sealing ability and less homogenous reparative dentin formation compared with primary dentin [1]. Several materials have been used in the past decades, such as zinc oxide eugenol, glass ionomer cement, adhesive resin, mineral trioxide aggregate, Biodentine, and enamel matrix derivatives, which have been shown to promote healing of pulp, whereas others do not have strong recommendations for use in clinical trials that indicate poor outcomes [15].
Since it was performed, many materials have been used for DPC, but what material should be ideal for DPC in dentin regeneration is still unclear. Numerous randomized and non-randomized studies with brief follow-up periods were carried out, which are insufficient to distinguish the long-term effects of different DPC-materials. By summarizing those studies, this review will recommend the most suitable materials for DPC in managing dentin regeneration, which will be useful information for dentists. The aim of this systematic review of the literature is to answer the research question: “Which biomaterial is more effective for dental capping in terms of dentin regeneration?”
2. Materials and Methods
2.1. Search Strategy
This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [16]. Five electronic databases were searched for articles: PubMed, Web of Science, Science Direct, Google Scholar, and Scopus. For the search, the following keyword combinations were used: (direct pulp capping [Title/Abstract]) AND (dentin regeneration [Title/Abstract]) AND (reparative dentin [Title/Abstract]) AND (dentin bridge formation [Title/Abstract]) AND (mineral trioxide aggregate [Title/Abstract]) OR (MTA [Title/Abstract]) AND (calcium hydroxide [Title/Abstract]) AND (biodentine [Title/Abstract]). All the authors reached a consensus on the search strategy. Then three independent authors pre-selected the articles as per titles and abstracts and submitted them for the other authors’ approval. After completing this extraction, four independent and experienced authors critically checked, extracted, and confirmed the data. Articles from 2005 to 2021 were reviewed, and the literature published until 2021 was systematically searched. The search encompassed articles (full text) that have been published in peer-reviewed journals and written in English related to DPC material used for dentin regeneration.
2.2. Study Selection
Here, the primary concern was finding out the type of DPC material, formation/regeneration of dentin, quality of dentin formation, and outcomes. The case reports and the letters to the editors were also excluded from this review. The titles and abstracts of identified studies were independently evaluated to ensure if the studies met the inclusion criteria.
2.3. Inclusion and Exclusion Criteria
Inclusion criteria included the following:
Clinical controlled trials (CCTs), randomized controlled trials (RCTs), and animal studies;
Studies on permanent teeth in clinical conditions;
Direct pulp capping.
Exclusion criteria included the following:
Indirect pulp capping;
Total pulpotomies;
Deciduous/primary dentition;
Studies with insufficient information;
Non-English publications.
2.4. Data Extraction and Organization
Data extraction was performed on the studies that met the inclusion criteria. The following data were collected: the first author’s name; the year of publication; the age range; sample size; where this research was carried out; type of teeth; intervention/control material used for DPC; and follow-up, finding, and outcomes. The data were extracted and double-checked by the four independent authors, using a standard format. Disagreements during data extraction were resolved by means of discussion and consensus by a fifth author (MKA).
2.5. Quality Assessment
The Cochrane collaboration’s tool [17] for human studies and SYRCLE’s risk of bias tool [18] for animal studies were used to assess the methodological quality. For human studies, the following 6 domains were assessed: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. For animal studies, the following 5 studies were assessed: sequence generation, baseline characteristics, blinding of outcome assessment, incomplete outcome data, and selective reporting. Using the Revman software, version 5.3, each domain was evaluated for a low, unclear, and high risk of bias.
3. Results
3.1. Selection of Studies
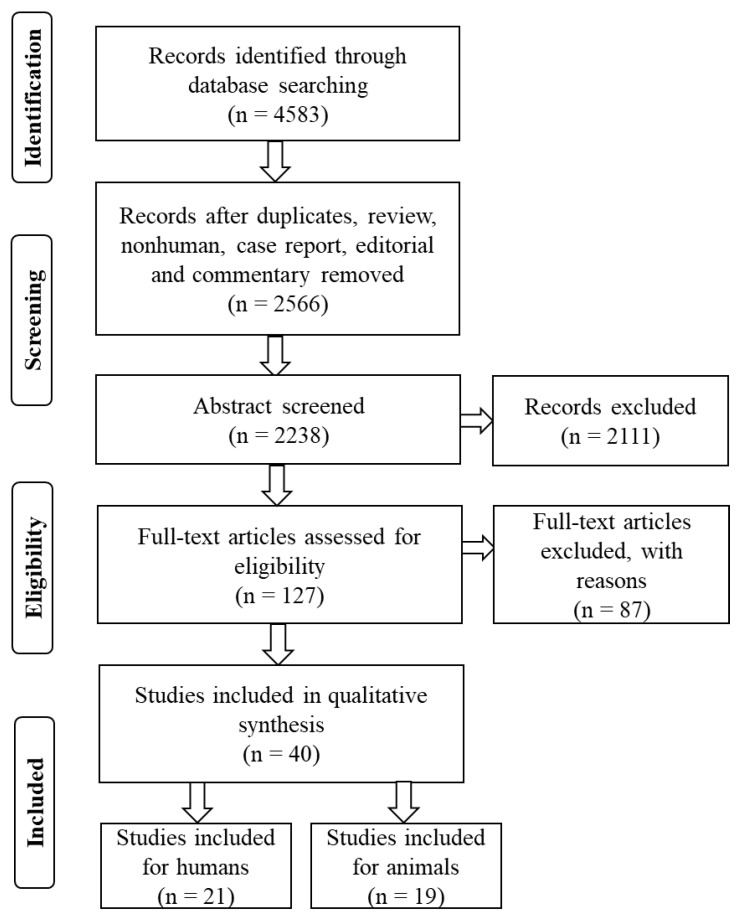
A total of 4583 papers from databases, including PubMed, Web of Science, Science Direct, Google Scholar, and Scopus, were initially identified by using this research search strategy. After removing the 2017 articles from consideration (duplicate studies, review, case repots, editorial letters, and comments), a second round of screening was conducted on the 2566 papers that remained. A total of 127 studies were considered worthy, and 87 studies were excluded because of an unacceptable data format. Thus, 40 studies (21 humans and 19 animals) were included in this study (Figure 1), with the complete text of all of the included studies being obtained based on the research goal and inclusion and exclusion criteria.
Figure 1.
Flowchart of the systematic review.
3.2. Study Characteristics
Table 1 summarizes the key characteristics of the human studies that were included in this systematic review. All of the included studies were journal articles and were conducted on adults. Among these 21 studies, five are from India, four from Brazil, three from Poland, and two from Egypt. Iran, Japan, China, Korea, UK, USA, and Turkey each had one study.
Table 1.
Characteristic of the studies included (humans) in the systematic review.
| Author (Year) | Country | Type of Study | Age | Type of Teeth | Experimental Materials | Comparing Materials | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Cobanoglu et al. 2021 [19] | Turkey | Controlled clinical trial | 23–35 | Third molars | Clearfil Protect Bond | Clearfil SE Bond and CH | 90 days | CH group showed better hard-tissue formation than the experimental group. |
| Sharma et al. 2021 [20] | India | Controlled clinical trial | 15–30 | Premolars | Endosequence Root Repair Material and Endocem MTA | ProRoot MTA | 30 days | The mean thickness of dentin-bridge formation in ProRoot MTA was greater than the other two experimental groups. |
| Holiel et al. 2021 [21] | Egypt | Controlled clinical trial | 15–25 | Premolars | Treated dentin matrix hydrogel | Biodentine and MTA | 2 weeks and 2 months | Complete dentin-bridge formation was observed with numerous dentinal tubule lines showing a positive trend to dentin regeneration. |
| Holiel et al. 2021 [22] | Egypt | Randomized clinical trial | 18–40 | Permanent posterior teeth | Treated dentin matrix hydrogel | Biodentine and MTA | 3, 6, 12, 18, and 24 months | Dentin-bridge formation was significantly superior of a higher thickness than Biodentine and MTA. |
| Hoseinifar et al. 2020 [23] | Iran | Randomized clinical trial | 14–25 | Premolars | Calcium-enriched mixture | MTA and Biodentine | 6 weeks | No significant differences were observed between the groups in terms of the dentine bridge formation. |
| Suzuki et al. 2019 [24] | Japan | Controlled clinical trial | 18–33 | Third molars | CO2 laser irradiation | Dycal | 6 and 12 months | Self-etching adhesive system following CO2 laser irradiation without carbonization of the exposed pulp demonstrated dentin-bridge formation that was comparable to Dycal. |
| Mahendran et al. 2019 [25] | India | Controlled clinical trial | 18–24 | Premolars | Simvastatin + α-TCP and atorvastatin + α-TCP | MTA | 7, 30, and 90 days | No significant difference was observed in terms of hard-tissue formation between the groups. |
| Jalan et al. 2017 [26] | India | Randomized clinical trial | 15–25 | Premolars | Biodentine | CH | 45 days | Dentin-bridge formation was significantly thicker and more continuous with Biodentine in comparison to Dycal. |
| Nowicka et al. 2016 [11] | Poland | Controlled clinical trial | 19–30 | Third molars | Single-bond universal | CH | 6 weeks | Single-bond universal showed less dentin-bridge formation than CH. |
| Nowicka et al. 2015 [27] | Poland | Controlled clinical trial | 19–32 | Third molars | MTA, Biodentine, single-bond universal | CH | 6 weeks | MTA and Biodentine groups showed significantly higher dentin-bridge formation than CH and single-bond universal groups. |
| Swarup et al. 2014 [28] | India | Controlled clinical trial | 11–15 | Premolars | Nano hydroxyapatite | MTA, CH | 15 and 30 days | Continuous dentin-bridge formation was observed in the nano hydroxyapatite and MTA groups. Only MTA group showed regular pattern of dentinal tubules. |
| Parolia et al. 2010 [29] | India | Controlled clinical trial | 15–25 | Premolars | Propolis, MTA | Dycal | 15 and 45 days | Propolis and MTA showed more dentin-bridge formation than Dycal group. |
| Accorinte et al. 2008 [30] | Brazil | Controlled clinical trial | 15–30 | Premolars | Clearfil LB 2V and Clearfil SE Bond | CH | 30 and 90 days | Few specimens showed dentin-bridge formation in the experimental group, whereas CH showed dentin-bridge formation almost all the specimens. |
| Accorinte et al. 2008 [31] | Brazil | Controlled clinical trial | 15–30 | Premolars | MTA | CH | 30 and 60 days | CH showed faster hard-tissue formation compared to MTA and a similar response with the hard-tissue bridge in almost all cases was observed. |
| Accorinte et al. 2008 [32] | Brazil | Controlled clinical trial | 15–30 | Premolars | MTA | CH | 30 and 60 days | Dentin-bridge formation was lower in the CH group compared to MTA group. |
| Sawicki et al. 2008 [33] | Poland | Controlled clinical trial | 10–18 | Immature premolars | WMTA | CH | 47–609 days | Complete, thicker, and more solid dentin bridge was observed in the WMTA group when compared with CH. |
| Lu et al. 2008 [34] | China | Controlled clinical trial | 20–25 | Third molars | Clearfil SE Bond | CH | 7, 30, and 90 days | The dentin-bridge formation in the experimental group was significantly lower compared to CH group. |
| Min et al. 2008 [35] | Korea | Controlled clinical trial | 21–50 | Third molars | MTA | CH | 2 months | The thickness of the dentin-bridge formation in the MTA group was statistically greater than CH group. |
| Nair et al. 2006 [36] | UK | Randomized controlled trial | 18–30 | Third molars | MTA | Dycal | 1 week, 1 month, and 3 months | Complete hard-tissue formation was observed in the MTA group, whereas less consistent formation of hard-tissue barrier with numerous tunnel defect was observed in the Dycal group. |
| Silva et al. 2006 [37] | Brazil | Controlled clinical trial | 12–20 | First premolars | Single-bond adhesive system | CH | 30 days | No dentin formation at the exposure area in the single-bong adhesive system group, whereas dentin-bridge formation was observed in the CH group. |
| Iwamoto et al. 2006 [38] | USA | Controlled clinical trial | 18–60 | Third molars | WMTA | CH | 136 ± 24 days | WMTA showed a dentin-bridge formation similar to CH’s. |
CH, calcium hydroxide; MTA, mineral trioxide aggregate; WMTA, white mineral trioxide aggregate; α-TCP, α-tricalcium phosphate.
Table 2 summarizes the key characteristics of the animal studies that were included in this systematic review. All of the included studies were journal articles and were conducted on animals. Among these 19 studies, five are from Japan, four from China, two Thailand, and two from Greece. Egypt, Korea, Portugal, Belgium, Tehran, and Germany had one each.
Table 2.
Characteristic of the studies included (animals) in the systematic review.
| Author (Year) | Country | Animal | Age/ Weight |
Type of Teeth | Experimental Materials | Comparing Materials | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Islam et al. 2021 [4] | Japan | Wister rats | 8–9 weeks | Maxillary first molar | Phosphorylated pullulan + MTA | MTA, Super Bond | 1, 3, 7, and 28 days | The experimental group showed more homogenous mineralized tissue formation compared to MTA and Super bond groups. |
| Yoon et al. 2021 [39] | Korea | Sprague-Dawley rats | 6–8 weeks | Maxillary first molar | Osteostatin + ProRoot MTA | ProRoot MTA | 4 weeks | The combined material group showed more mineralized dentin-bridge formation compared to ProRoot MTA group. |
| Trongkij et al. 2019 [40] | Thailand | Wister rats | 8 weeks | Maxillary first molar | Bio-MA | WMTA | 1, 3, and 30 days | Complete dentin-bridge formation was observed in the Bio-MA group which is similar to WMTA. |
| Hanada et al. 2019 [41] | Japan | Wister rats | 9 weeks | Maxillary first molar | Bioactive glass | Dycal and WMTA | 1, 4, and 7 days | Bioactive-glass-based cement induced a significant level of reparative dentin formation, similar to MTA. |
| Takahashi et al. 2019 [42] | Japan | Wister rats | 9 weeks | Maxillary first molars | S-PRG filler | MTA | 1, 2, and 4 weeks | S-PRG filler showed to promote tertiary dentinogenesis, which is similar to MTA. |
| Paula et al. 2019 [43] | Portugal | Wister rats | 12–14 weeks | First mandibular molars | WMTA and Biodentine | Positive control (exposure without treatment) | 3, 7, and 21 days | Mineralized tissue formation was observed in the WMTA and Biodentine group. Biodentine may lead to the formation of pulp calcifications. |
| Li et al. 2018 [44] | Belgium | Minipigs | 33–35 months | Incisors, canines, premolars and molars | Tricalcium silicate cement | ProRoot MTA and TheraCal | 70 days | Complete reparative dentin formation with tubular structures was observed in the tricalcium silicate and ProRoot MTA groups. |
| Trongkij et al. 2018 [45] | Thailand | Wister rats | 8 weeks | Maxillary first molar | Bio-MA | WMTA | 1 and 7 days | Bio-MA can stimulate reparative dentin formation which is similar to WMTA. |
| Shinkai et al. 2017 [46] | Japan | Sprague-Dawley rats | 8–9 weeks | Maxillary first molar | All-in-one adhesives (Clearfil Tri-SBond ND, G Bond Plus, Bond Force, Adper Easy Bond, Xeno V) | MTA | 14 days | MTA group showed complete dentin-bridge formation, whereas all-in-one adhesives group showed incomplete or partial dentin-bridge formation. |
| Negm et al. 2017 [47] | Egypt | Dogs | 4–6 months | Four teeth in three quadrants | Portland cement + 10% calcium hydroxide + 20% bismuth oxide, Portland cement + bismuth oxide | MTA | 3 weeks and 3 months | Addition of calcium hydroxide to Portland cement improves the dentin-bridge formation qualitatively and quantitatively. |
| Shi et al. 2016 [48] | China | Beagle dogs | 8 months | Maxillary and mandibular incisors | iRoot BP Plus | MTA | 3 months | Both experimental groups showed complete calcified bridge formation with no significant difference. |
| Suzuki et al. 2016 [49] | Japan | Sprague-Dawley rats | 6 weeks | Maxillary first molar | Adhesive resin – Primer I, II and III | Dycal | 14, 28, 56, and 112 days | Higher quality of the mineralized tissue formation was observed in the experimental groups. |
| Liu et al. 2015 [50] | China | Wister rats | 180–200 g | Maxillary first molars | iRoot BP Plus | MTA | 1 and 4 weeks | iRoot BP Plus induced the formation of reparative dentin bridge. |
| Tziafa et al. 2014 [51] | Greece | Miniature swine | 18 months | Incisors, canines, premolars and molars | Biodentine | MTA angelus | 3 and 8 weeks | The thickness of hard-tissue bridge formation was significantly higher in the Biodentine group. |
| Danesh et al. 2012 [52] | Tehran | Dogs | 18–24 months | Canine | Biomimetic carbonated apatite | MTA | 7 and 70 days | Biomimetic carbonated apatite did not induce hard-tissue bridge formation. |
| Dammaschke et al. 2010 [53] | Germany | Wister rats | 3 months | Maxillary first molars | Reculcin AquaPrime+ monoBond, ScotchBond 1, Gluma Comfort Bond | CH | 1, 3, 7, and 70 days | CH showed more frequent reparative dentin formation than the experimental groups. |
| Cui et al. 2009 [54] | China | Dog | 1.5 years | Incisor, canine, premolars and first molar | Clearfil SE Bond, Imperva FluoroBond, Prompt L-Pop |
Dycal | 7, 14, and 30 days | Hard-tissue formation was observed in the experimental group. |
| Lu et al. 2006 [55] | China | Beagles | 1 year | All teeth | Clearfil SE Bond | CH | 7, 30, and 90 days | Dentin-bridge formation was less in the experimental group than CH. |
| Koliniotou-Koumpia and Tziafas 2005 [56] | Greece | Dog | 2.5–3.5 years | Maxillary and mandibulary molars, premolars, canines, and third incisors | Clearfil SE bond, Prompt L-pop, Etch and prime 3.0, single-bond | Dycal | 7, 21, and 65 days | Continuous hard-tissue bridge formation was totally absence in the experimental groups. |
CH, calcium hydroxide; MTA, mineral trioxide aggregate; WMTA, white mineral trioxide aggregate; CaCl2, calcium chloride.
3.3. Risk of Bias
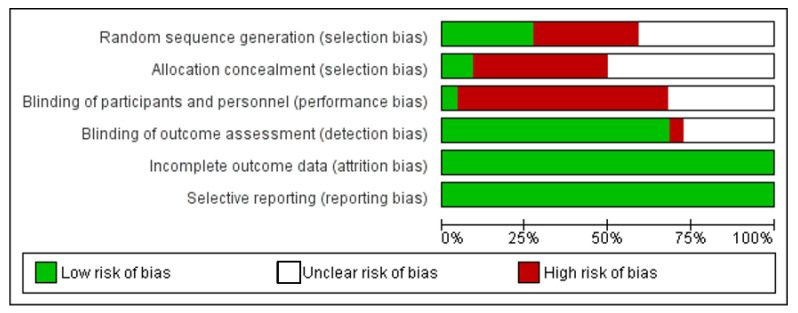
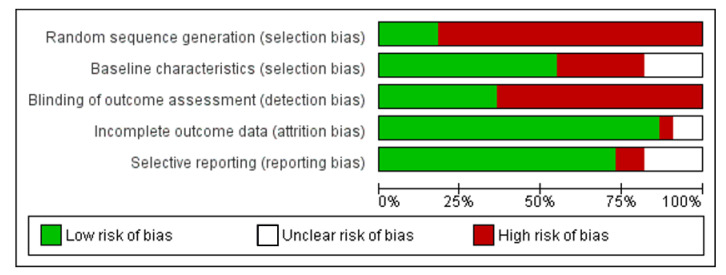
Figure 2 summarizes the assessment of the risk of bias in the included human studies. All assessed studies exhibited low attrition and reporting bias, whereas selection bias (random sequence and allocation concealment) and performance bias had a high and unclear risk of bias. Figure 3 summarizes the assessment of the risk of bias in the included animal studies. Studies exhibited low selection (baseline characteristics), attrition, and reporting bias, whereas selection bias (random sequence) and detection bias had a high risk of bias.
Figure 2.
Risk-of-bias graph: review authors’ judgement about each risk of bias item presented as percentage across all included human studies.
Figure 3.
Risk-of-bias graph: review authors’ judgement about each risk of bias item presented as percentage across all included animal studies.
4. Discussion
The aim of this current systematic review was to assess the efficacy of various DPC materials that are used in dentin regeneration. This systematic review employed a risk of bias assessment of the included studies, which revealed that some of them had poor methodological quality. These studies investigated different age groups, gender, and tooth type at the population level. The quality of the included studies ranged from low to moderate, and many of them were associated with a high risk of bias. The primary goal of DPC is to maintain the pulpal tissue’s full integrity under various pathological conditions of exposure [57]. An ideal DPC material should not cause pulpal inflammation, which can lead to necrosis, and should regenerate good quality dentin at the exposure area [4]. It has been demonstrated that the use of calcium silicate–based materials as DPC agents can effectively treat dental pulp. CH has long been regarded as the gold standard of DPC material because of its biocompatibility, high pH, antibacterial effect, and ability to form a new dentin bridge at the exposure site [57]. The use of CH as a DPC material was proven to have a higher clinical success rate according to studies that followed patients for more than 10 years [58]. CH has high alkalinity, which leads to necrosis and inflammation to the pulp [9]. Besides its high solubility and lack of adhesion with hard tissues, it does not provide an optimal seal, even though the dentin bridge appears to be fully formed by the time of its complete dissolution [59,60]. CH presents tunnel defects in the dentin bridge, but there is evidence to suggest that the appearance of these defects improves with increased dentin-bridge thickness [57]. In comparison to CH, MTA has a higher rate of clinical success and can result in dentin-bridge formation that is much thicker [61,62]. Based on calcium oxide, CH and MTA both react to carbon dioxide in tissues, which is a similar mechanism of action. At the exposure site, calcite granulations are formed, and fibronectin accumulates, promoting cellular migration, proliferation, adhesion, and differentiation, resulting in the formation of hard tissue [57,63]. Bioactive molecules are released during this process that facilitate regeneration of dental pulp and are integrated into the dentin matrix during the process of dentinogenesis [64,65,66].
Nowadays, MTA has proven to be a suitable choice for DPC material because of its good sealing ability and biocompatibility [4]. Studies demonstrated that dentinal-bridge formation by MTA was higher quality, less porous, thicker, and caused less pulpal inflammation than CH [3]. Furthermore, MTA has been demonstrated to induce adhesion, migration, and attachment of undifferentiated cells in order to form a dentinal bridge while having minimal inflammatory effect on the pulp [5,67]. However, the MTA has some disadvantages, including high cost, difficulty in handling, and long setting time [4]. In a practice-based research network, confirmatory evidence for MTA’s superior performance as a DPC agent emerged when it was compared to CH in a randomized clinical trial [68]. The dental pulp capped with MTA had a 92.5 to 97.96 percent success rate in clinical trials, according to a review of the few clinical observations [69,70]. According to a histological study, MTA application directly affects the dental pulp’s regeneration potential and is associated with an increase in TGF-1 secretion by the pulp cells [71]. These cells migrate to the material–pulp interface, where they are stimulated to differentiate into odontoblastic cells, which secrete reparative dentin, affecting the quality of the dentin-bridge formation [72]. Further histological examination revealed that the hard-tissue barrier formation after DPC with MTA is not the result of the differentiation of true odontoblast and does not have the properties of regular dentin [73]. These findings recommend that the calcified tissue formation should be considered as a reparative process rather than a real regeneration process. As a result, regular dentin could not be regenerated, and a fast-setting pulp-capping material could not be used in regenerative dentistry, due to its inadequate bioactive potential [72]. Furthermore, MTA and Biodentine, in contrast to calcium hydroxide, have favorable metabolic activity and stimulate almost similar desired cellular response, resulting in a higher rate of clinical success [74]. When comparing the MTA and Biodentine groups in terms of the formation of dentin bridge, micro-CT imaging demonstrated that the MTA group had a more regular pattern of reparative dentin layer which is homogenous and uniform thickness. These findings revealed that both MTA and Biodentine have the ability to induce the dentin-bridge formation, with MTA being the most effective at improving the quality of dentin [75]. Therefore, MTA is the preferred material for DPC [72].
Biodentine is a newer calcium silicate–based DPC material having properties similar to CH and MTA, as well as favorable effects on the dental-pulp cells that promote the formation of tertiary reparative dentin [62]. By releasing TGF-β1 and stimulating odontoblasts, Biodentine promotes pulpal healing and mineralization [3,76]. Biodentine also releases silicon ions that play a significant role during the process of mineralizing the dentinal bridge [3]. It has been demonstrated that the formation of the dentin bridge by Biodentine is similar to that of MTA with no pulpal inflammatory response [77]. This is due to the anti-inflammatory effect, which inhibits the secretion of pro-inflammatory substances and reduces the recruitment of inflammatory cells [77]. According to Nowicka et al.’s findings, Biodentine and MTA induced homogeneous reparative dentin formation, whereas CH induced a more porous formation, implying that calcium silicates induce higher tissue-repair efficacy as compared to CH [27]. Jalan et al. found similar superior outcomes for dental pulp capped with Biodentine when compared to Dycal [26]. Therefore, Biodentine material has great potential as a pulp-capping agent because of its proper setting time and restorative properties. However, studies suggested that long-term clinical research is still required to check the efficacy of Biodentine [3].
Adhesive systems have been investigated as suitable DPC materials because of their ability to adhere to dentin to protect the pulp from bacterial contamination [1]. On the other hand, bonding agents have been shown to have direct cytotoxic effects on dental-pulp cells [1]. These materials did not show favorable responses when compared to MTA in terms of pulpal inflammation and hard-tissue formation [57]. A new dentin-bridge formation was observed in all MTA specimens, whereas no hard-tissue deposition was observed even if the pulp tissue showed no symptoms of inflammation in the polymeric-based materials group, or the adhesive materials group only induced a few hard-tissue depositions with pulpal necrosis and inflammation [78,79].
5. Conclusions
The findings of this systematic review, based on the available information, conclude that MTA and its variants have a higher success rate in dentin regeneration. MTA and its variants are more likely to form a homogenous dentinal bridge than CH and other DPC materials.
Author Contributions
Conceptualization, E.N., J.Y., and R.I.; methodology, E.N., J.Y. and R.J.; validation, J.Y., R.I. and X.L. (Xiang Li); investigation, J.Y., X.L. (Xiangzhen Liu) and M.K.A.; writing—original draft preparation, E.N., J.Y. and R.I.; writing—review and editing, X.L. (Xiangzhen Liu) and M.K.A.; supervision, J.Y. and M.K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors would like to exclude the data availability statement.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Didilescu A.C., Cristache C.M., Andrei M., Voicu G., Perlea P. The effect of dental pulp-capping materials on hard-tissue barrier formation: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2018;149:903–917. doi: 10.1016/j.adaj.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Kawashima N., Okiji T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom. 2016;56:144–153. doi: 10.1111/cga.12169. [DOI] [PubMed] [Google Scholar]
- 3.Davaie S., Hooshmand T., Ansarifard S. Different types of bioceramics as dental pulp capping materials: A systematic review. Ceram. Int. 2021;47:20781–20792. doi: 10.1016/j.ceramint.2021.04.193. [DOI] [Google Scholar]
- 4.Islam R., Toida Y., Chen F., Tanaka T., Inoue S., Kitamura T., Yoshida Y., Chowdhury A.F.M.A., Ahmed H.M.A., Sano H. Histological evaluation of a novel phosphorylated pullulan-based pulp capping material: An in vivo study on rat molars. Int. Endod. J. 2021;54:1902–1914. doi: 10.1111/iej.13587. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L., Yang J., Zhang J., Peng B. A comparative study of BioAggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. J. Endod. 2014;40:1118–1123. doi: 10.1016/j.joen.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Toida Y., Kawano S., Islam R., Jiale F., Chowdhury A.A., Hoshika S., Shimada Y., Tagami J., Yoshiyama M., Inoue S., et al. Pulpal response to mineral trioxide aggregate containing phosphorylated pullulan-based capping material. Dent. Mater. J. :2021. doi: 10.4012/dmj.2021-153. [DOI] [PubMed] [Google Scholar]
- 7.Çalışkan M.K., Güneri P. Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2-to 6-year follow-up. Clin. Oral Investig. 2017;21:357–367. doi: 10.1007/s00784-016-1798-z. [DOI] [PubMed] [Google Scholar]
- 8.Mente J., Hufnagel S., Leo M., Michel A., Gehrig H., Panagidis D., Panagidis D., Saure D., Pfefferle T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014;40:1746–1751. doi: 10.1016/j.joen.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Hilton T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009;34:615–625. doi: 10.2341/09-132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utneja S., Nawal R.R., Talwar S., Verma M. Current perspectives of bio-ceramic technology in endodontics: Calcium enriched mixture cement-review of its composition, properties and applications. Restor. Dent. Endod. 2015;40:1–13. doi: 10.5395/rde.2015.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicka A., Łagocka R., Lipski M., Parafiniuk M., Grocholewicz K., Sobolewska E., Witek A., Buczkowska-Radlińska J. Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. BioMed Res. Int. 2016;2016:2591273. doi: 10.1155/2016/2591273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes A.M., Silva G.A., Lopes Jr N., Napimoga M.H., Benatti B.B., Alves J.B. Direct capping of human pulps with a dentin bonding system and calcium hydroxide: An immunohistochemical analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;105:385–390. doi: 10.1016/j.tripleo.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Hörsted-Bindslev P., Vilkinis V., Sidlauskas A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;96:591–600. doi: 10.1016/S1079-2104(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 14.Morotomi T., Washio A., Kitamura C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019;55:5–11. doi: 10.1016/j.jdsr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwendicke F., Brouwer F., Schwendicke A., Paris S. Different materials for direct pulp capping: Systematic review and meta-analysis and trial sequential analysis. Clin. Oral Investig. 2016;20:1121–1132. doi: 10.1007/s00784-016-1802-7. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobanoglu N., AlptekIn T., Kitagawa H., Blatz M.B., Imazato S., Ozer F. Evaluation of human pulp tissue response following direct pulp capping with a self-etching adhesive system containing MDPB. Dent. Mat. J. 2021;40:689–696. doi: 10.4012/dmj.2020-145. [DOI] [PubMed] [Google Scholar]
- 20.Sharma V., Nawal R.R., Augustine J., Urs A.B., Talwar S. Evaluation of Endosequence Root Repair Material and Endocem MTA as direct pulp capping agents: An in vivo study. Aust. Endod. J. 2021 doi: 10.1111/aej.12542. [DOI] [PubMed] [Google Scholar]
- 21.Holiel A.A., Mahmoud E.M., Abdel-Fattah W.M., Kawana K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2021;25:2101–2112. doi: 10.1007/s00784-020-03521-z. [DOI] [PubMed] [Google Scholar]
- 22.Holiel A.A., Mahmoud E.M., Abdel-Fattah W.M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 2021;25:4621–4634. doi: 10.1007/s00784-021-03775-1. [DOI] [PubMed] [Google Scholar]
- 23.Hoseinifar R. Histological Evaluation of Human Pulp Response to Direct Pulp Capping with MTA, CEM Cement, and Biodentine. J. Dent. 2020;21:177–183. doi: 10.30476/DENTJODS.2019.81796.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M., Kato C., Kawashima S., Shinkai K. Clinical and histological study on direct pulp capping with CO2 laser irradiation in human teeth. Oper Dent. 2019;44:336–347. doi: 10.2341/18-030-C. [DOI] [PubMed] [Google Scholar]
- 25.Mahendran K., Ponnusamy C., Maloor S.A. Histological evaluation of pulpal response to direct pulp capping using statins with α-tricalcium phosphate and mineral trioxide aggregate in human teeth. J. Conserv. Dent. 2019;22:441. doi: 10.4103/JCD.JCD_418_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalan A.L., Warhadpande M.M., Dakshindas D.M. A comparison of human dental pulp response to calcium hydroxide and Biodentine as direct pulp-capping agents. J. Conserv. Dent. 2017;20:129–133. doi: 10.4103/0972-0707.212247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowicka A., Wilk G., Lipski M., Kołecki J., Buczkowska-Radlińska J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca (OH) 2, MTA, Biodentine, and dentin bonding system in human teeth. J. Endod. 2015;41:1234–1240. doi: 10.1016/j.joen.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Swarup S.J., Rao A., Boaz K., Srikant N., Shenoy R. Pulpal response to nano hydroxyapatite, mineral trioxide aggregate and calcium hydroxide when used as a direct pulp capping agent: An in vivo study. J. Clin. Pediatr Dent. 2014;38:201–206. doi: 10.17796/jcpd.38.3.83121661121g6773. [DOI] [PubMed] [Google Scholar]
- 29.Parolia A., Kundabala M., Rao N.N., Acharya S.R., Agrawal P., Mohan M., Thomas M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust. Dent. J. 2010;55:59–64. doi: 10.1111/j.1834-7819.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 30.Accorinte M.L.R., Loguercio A.D., Reis A., Costa C.A.D.S. Response of human pulps capped with different self-etch adhesive systems. Clin. Oral Investig. 2008;12:119–127. doi: 10.1007/s00784-007-0161-9. [DOI] [PubMed] [Google Scholar]
- 31.Accorinte M.L.R., Loguercio A.D., Reis A., Carneiro E., Grande R.H.M., Murata S.S., Holland R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper. Dent. 2008;33:488–495. doi: 10.2341/07-143. [DOI] [PubMed] [Google Scholar]
- 32.Accorinte M.L.R., Holland R., Reis A., Bortoluzzi M.C., Murata S.S., Dezan E., Jr., Souza V., Alessandro L.D. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J. Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Sawicki L., Pameijer C.H., Emerich K., Adamowicz-Klepalska B. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am. J. Dent. 2008;21:262–266. [PubMed] [Google Scholar]
- 34.Lu Y., Liu T., Li H., Pi G. Histological evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide on human pulp tissue. Int. Endod. J. 2008;41:643–650. doi: 10.1111/j.1365-2591.2008.01396.x. [DOI] [PubMed] [Google Scholar]
- 35.Min K.S., Park H.J., Lee S.K., Park S.H., Hong C.U., Kim H.W., Lee H.H., Kim E.C. Effect of mineral trioxide aggregate on dentin bridge formation and expression of dentin sialoprotein and heme oxygenase-1 in human dental pulp. J. Endod. 2008;34:666–670. doi: 10.1016/j.joen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Nair P.N.R., Duncan H.F., Pitt Ford T.R., Luder H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008;41:128–150. doi: 10.1111/j.1365-2591.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 37.Silva G.A., Lanza L.D., Lopes-Júnior N., Moreira A., Alves J.B. Direct pulp capping with a dentin bonding system in human teeth: A clinical and histological evaluation. Oper. Dent. 2006;31:297–307. doi: 10.2341/05-65. [DOI] [PubMed] [Google Scholar]
- 38.Iwamoto C.E., Adachi E., Pameijer C.H., Barnes D., Romberg E.E., Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am. J. Dent. 2006;19:85–90. [PubMed] [Google Scholar]
- 39.Yoon J.H., Choi S.H., Koh J.T., Lee B.N., Chang H.S., Hwang I.N., Oh W.M., Hwang Y.C. Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo. Restor. Dent. Endod. 2021;46:e17. doi: 10.5395/rde.2021.46.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trongkij P., Sutimuntanakul S., Lapthanasupkul P., Chaimanakarn C., Wong R.H., Banomyong D. Pulpal responses after direct pulp capping with two calcium-silicate cements in a rat model. Dent. Mat. J. 2019;38:584–590. doi: 10.4012/dmj.2018-225. [DOI] [PubMed] [Google Scholar]
- 41.Hanada K., Morotomi T., Washio A., Yada N., Matsuo K., Teshima H., Yokota K., Kitamura C. In vitro and in vivo effects of a novel bioactive glass- based cement used as a direct pulp capping agent. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:161–168. doi: 10.1002/jbm.b.34107. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi Y., Okamoto M., Komichi S., Imazato S., Nakatsuka T., Sakamoto S., Kimoto K., Hayashi M. Application of a direct pulp capping cement containing S- PRG filler. Clin. Oral Investig. 2019;23:1723–1731. doi: 10.1007/s00784-018-2596-6. [DOI] [PubMed] [Google Scholar]
- 43.Paula A.B., Laranjo M., Marto C.M., Paulo S., Abrantes A.M., Fernandes B., Casalta-Lopes J., Marques-Ferreira M., Botelho M.F., Carrilho E. Evaluation of dentinogenesis inducer biomaterials: An in vivo study. J. Appl. Oral Sci. 2019;28:e20190023. doi: 10.1590/1678-7757-2019-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Pedano M.S., Camargo B., Hauben E., De Vleeschauwer S., Chen Z., Munck J.D., Vandamme K., Landuyt K.V., Meerbeek B.V. Experimental tricalcium silicate cement induces reparative dentinogenesis. Dent. Mater. 2018;34:1410–1423. doi: 10.1016/j.dental.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Trongkij P., Sutimuntanakul S., Lapthanasupkul P., Chaimanakarn C., Wong R., Banomyong D. Effects of the exposure site on histological pulpal responses after direct capping with 2 calcium- silicate based cements in a rat model. Restor. Dent. Endod. 2018;43:e36. doi: 10.5395/rde.2018.43.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinkai K., Taira Y., Kawashima S., Suzuki S., Suzuki M. Histological evaluation of direct pulp capping with all- in- one adhesives in rat teeth. Dent. Mat. J. 2017;36:348–356. doi: 10.4012/dmj.2016-148. [DOI] [PubMed] [Google Scholar]
- 47.Negm A.M., Hassanien E.E., Abu-Seida A.M., Nagy M.M. Biological evaluation of a new pulp capping material developed from Portland cement. Exp. Toxicol. Pathol. 2017;69:115–122. doi: 10.1016/j.etp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Shi S., Bao Z.F., Liu Y., Zhang D.D., Chen X., Jiang L.M., Zhong M. Comparison of in vivo dental pulp responses to capping with iRoot BP Plus and mineral trioxide aggregate. Int. Endod. J. 2016;49:154–160. doi: 10.1111/iej.12439. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M., Taira Y., Kato C., Shinkai K., Katoh Y. Histological evaluation of direct pulp capping of rat pulp with experimentally developed low-viscosity adhesives containing reparative dentin-promoting agents. J. Dent. 2016;44:27–36. doi: 10.1016/j.jdent.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Liu S., Wang S., Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 2015;41:652–657. doi: 10.1016/j.joen.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Tziafa C., Koliniotou-Koumpia E., Papadimitriou S., Tziafas D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J. Endod. 2014;40:1967–1971. doi: 10.1016/j.joen.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Danesh F., Vahid A., Jahanbani J., Mashhadiabbas F., Arman E. Effect of white mineral trioxide aggregate compared with biomimetic carbonated apatite on dentine bridge formation and inflammatory response in a dental pulp model. Int. Endod. J. 2012;45:26–34. doi: 10.1111/j.1365-2591.2011.01943.x. [DOI] [PubMed] [Google Scholar]
- 53.Dammaschke T., Stratmann U., Fischer R.J., Sagheri D., Schäfer E. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. 2010;41:62–71. [PubMed] [Google Scholar]
- 54.Cui C., Zhou X., Chen X., Fan M., Bian Z., Chen Z. The adverse effect of self-etching adhesive systems on dental pulp after direct pulp capping. Quintessence Int. 2009;40:26–34. [PubMed] [Google Scholar]
- 55.Lu Y., Liu T., Li X., Li H., Pi G. Histologic evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide in beagles. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:78–84. doi: 10.1016/j.tripleo.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Koliniotou-Koumpia E., Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J. Dent. 2005;33:639–647. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Andrei M., Vacaru R.P., Coricovac A., Ilinca R., Didilescu A.C., Demetrescu I. The Effect of Calcium-Silicate Cements on Reparative Dentinogenesis Following Direct Pulp Capping on Animal Models. Molecules. 2021;26:2725. doi: 10.3390/molecules26092725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair M., Gurunathan D. Clinical and radiographic outcomes of calcium hydroxide vs other agents in indirect pulp capping of primary teeth: A systematic review. Int. J. Clin. Pediatr. Dent. 2019;12:437–444. doi: 10.5005/jp-journals-10005-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrou M.A., Alhamoui F.A., Welk A., Altarabulsi M.B., Alkilzy M., Splieth C.H. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin. Oral Investig. 2014;18:1383–1389. doi: 10.1007/s00784-013-1107-z. [DOI] [PubMed] [Google Scholar]
- 60.Li Z., Cao L., Fan M., Xu Q. Direct Pulp Capping with Calcium Hydroxide or Mineral Trioxide Aggregate: A Meta-analysis. J. Endod. 2015;41:1412–1417. doi: 10.1016/j.joen.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Zaen El-Din A.M., Hamama H.H., Abo El-Elaa M.A., Grawish M.E., Mahmoud S.H., Neelakantan P. The effect of four materials on direct pulp capping: An animal study. Aust. Endod. J. 2020;46:249–256. doi: 10.1111/aej.12400. [DOI] [PubMed] [Google Scholar]
- 62.Tran X.V., Gorin C., Willig C., Baroukh B., Pellat B., Decup F., Opsahl Vital S., Chaussain C., Boukpessi T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012;91:1166–1171. doi: 10.1177/0022034512460833. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005;16:369–376. doi: 10.1016/j.cytogfr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Graham L., Cooper P.R., Cassidy N., Nor J.E., Sloan A.J., Smith A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006;27:2865–2873. doi: 10.1016/j.biomaterials.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Duque C., Hebling J., Smith A.J., Giro E.M., Oliveira M.F., de Souza Costa C.A. Reactionary dentinogenesis after applying restorative materials and bioactive dentin matrix molecules as liners in deep cavities prepared in nonhuman primate teeth. J. Oral Rehabil. 2006;33:452–461. doi: 10.1111/j.1365-2842.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W., Walboomers X.F., Jansen J.A. The formation of tertiary dentin after pulp capping with a calcium phosphate cement, loaded with PLGA microparticles containing TGF-beta1. J. Biomed. Mater. Res. A. 2008;85:439–444. doi: 10.1002/jbm.a.31558. [DOI] [PubMed] [Google Scholar]
- 67.Zhu C., Ju B., Ni R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: A systematic review and meta-analysis. Int J. Clin. Exp. Med. 2015;8:17055–17060. [PMC free article] [PubMed] [Google Scholar]
- 68.Hilton T.J., Ferracane J.L., Mancl L. Comparison of CaOH with MTA for Direct Pulp Capping: A PBRN Randomized Clinical Trial. J. Dent. Res. 2013;92:16–22. doi: 10.1177/0022034513484336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniele L. Mineral Trioxide Aggregate (MTA) direct pulp capping: 10 years clinical results. G Ital. Endod. 2017;31:48–57. doi: 10.1016/j.gien.2017.04.003. [DOI] [Google Scholar]
- 70.Bogen G., Kim J.S., Bakland L.K. Direct pulp capping with mineral trioxide aggregate: An observational study. J. Am. Dent. Assoc. 2008;139:305–315. doi: 10.14219/jada.archive.2008.0160. [DOI] [PubMed] [Google Scholar]
- 71.Laurent P., Camps J., About I. BiodentineTM induces TGF-1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012;45:439–448. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 72.Kunert M., Lukomska-Szymanska M. Bio-inductive materials in direct and indirect pulp capping—A review article. Materials. 2020;13:1204. doi: 10.3390/ma13051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dammaschke T., Nowicka A., Lipski M., Ricucci D. Histological evaluation of hard tissue formation after direct pulp capping with a fast-setting mineral trioxide aggregate (RetroMTA) in humans. Clin. Oral Investig. 2019;23:4289–4299. doi: 10.1007/s00784-019-02876-2. [DOI] [PubMed] [Google Scholar]
- 74.Paula A., Laranjo M., Marto C.M., Abrantes A.M., Casalta-Lopes J., Gonçalves A.C., Sarmento-Ribeiro A.B., Ferreira M.M., Botelho M.F., Carrilho E. Biodentine™ boosts, WhiteProRoot® MTA increases and Life® suppresses odontoblast activity. Materials. 2019;12:1184. doi: 10.3390/ma12071184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J., Song Y.S., Min K.S., Kim S.H., Koh J.T., Lee B.N., Chang H.S., Hwang I.N., Oh W.M., Hwang Y.C. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor. Dent. Endod. 2016;41:29. doi: 10.5395/rde.2016.41.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giraud T., Jeanneau C., Rombouts C., Bakhtiar H., Laurent P., About I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019;35:24–35. doi: 10.1016/j.dental.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Giraud T., Jeanneau C., Bergmann M., Laurent P., About I. Tricalcium silicate capping materials modulate pulp healing and inflammatory activity in vitro. J. Endod. 2018;44:1686–1691. doi: 10.1016/j.joen.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Long Y., Liu S., Zhu L., Liang Q., Chen X., Dong Y. Evaluation of pulp response to novel bioactive glass pulp cap-ping materials. J. Endod. 2017;43:1647–1650. doi: 10.1016/j.joen.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 79.Simon S., Cooper P., Smith A., Picard B., Ifi C.N., Berdal A. Evaluation of a new laboratory model for pulp healing: Preliminary study. Int. Endod. J. 2008;41:781–790. doi: 10.1111/j.1365-2591.2008.01433.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors would like to exclude the data availability statement.