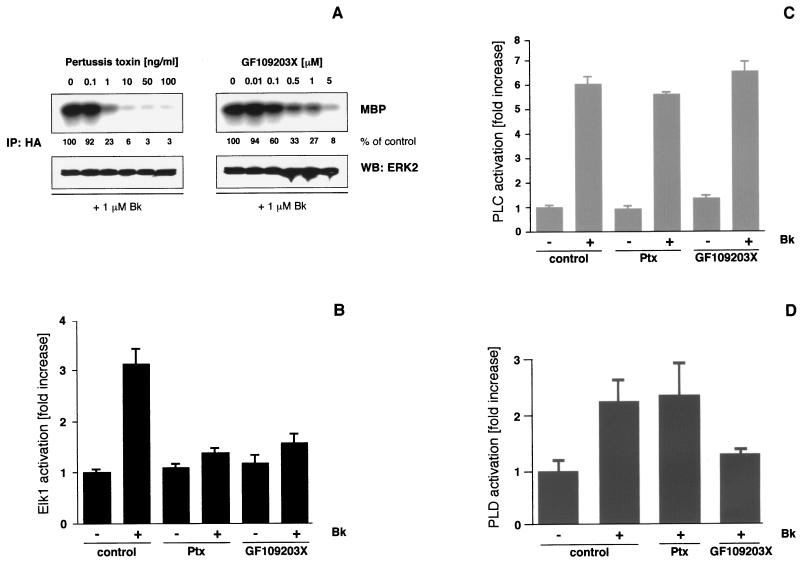
FIG. 1.
Effects of Ptx and PKC inhibition on the B2R-mediated signal transduction in HEK293T cells. (A) HEK293T cells grown on six-well plates were transiently transfected with B2R (1 μg/well) and HA-ERK2 (0.5 μg/well) using the Lipofectamine method (6 μl/well). After 24 h of serum starvation and 16 h of Ptx or 30 min of GF109203X pretreatment with the indicated concentrations, 1 μM bradykinin (Bk) was applied for 5 min. Cells were lysed, and ERK2 activity was determined after immunoprecipitation (IP) with a polyclonal anti-HA antibody using MBP as a substrate. Western blots (WB) with polyclonal ERK2 antibodies (0.5 μg/ml) were performed to confirm that equal amounts of ERK2 were present in the precipitates, and phosphorylated MBP was detected by autoradiography and quantified by phosphorimager analysis (Fuji BAS2000). MBP phosphorylation is expressed as a percentage of the signal obtained in the particular experiment in the absence of any inhibitor. In general, the background of basal ERK activity was about 2 to 10% of the signal seen with agonist stimulation. Representative autoradiograms and blots from three experiments with identical results are shown. (B) To analyze Elk1 activation, a reporter gene assay was used. Luciferase activities in cells stimulated with 1 μM bradykinin for 12 h were measured and compared with those obtained after pretreatment with Ptx (50 ng/ml, 16 h) or GF109203X (5 μM, 30 min) prior to agonist challenge. Means ± the standard deviation (SD) of three independent experiments performed in triplicates are shown. (C and D) In B2R-transfected HEK293T cells grown on 12-well plates, the increase of total inositol phosphates (PLC activation) and the production of phosphatidic acid, trapped as [3H]phosphatidylbutanol (PLD activation), were measured without or with 10 min of 1 μM bradykinin stimulation. Results were compared with those obtained under identical conditions but with inhibitor pretreatment prior to bradykinin challenge. Means ± the SD of triplicates from typical experiments are shown.