Abstract
Background: Coronavirus disease 2019 (COVID-19), a global pandemic, has caused over 216 million cases and 4.50 million deaths as of 30 August 2021. Vaccines can be regarded as one of the most powerful weapons to eliminate the pandemic, but the impact of vaccines on daily COVID-19 cases and deaths by country is unclear. This study aimed to investigate the correlation between vaccines and daily newly confirmed cases and deaths of COVID-19 in each country worldwide. Methods: Daily data on firstly vaccinated people, fully vaccinated people, new cases and new deaths of COVID-19 were collected from 187 countries. First, we used a generalized additive model (GAM) to analyze the association between daily vaccinated people and daily new cases and deaths of COVID-19. Second, a random effects meta-analysis was conducted to calculate the global pooled results. Results: In total, 187 countries and regions were included in the study. During the study period, 1,011,918,763 doses of vaccine were administered, 540,623,907 people received at least one dose of vaccine, and 230,501,824 people received two doses. For the relationship between vaccination and daily increasing cases of COVID-19, the results showed that daily increasing cases of COVID-19 would be reduced by 24.43% [95% CI: 18.89, 29.59] and 7.50% [95% CI: 6.18, 8.80] with 10,000 fully vaccinated people per day and at least one dose of vaccine, respectively. Daily increasing deaths of COVID-19 would be reduced by 13.32% [95% CI: 3.81, 21.89] and 2.02% [95% CI: 0.18, 4.16] with 10,000 fully vaccinated people per day and at least one dose of vaccine, respectively. Conclusions: These findings showed that vaccination can effectively reduce the new cases and deaths of COVID-19, but vaccines are not distributed fairly worldwide. There is an urgent need to accelerate the speed of vaccination and promote its fair distribution across countries.
Keywords: COVID-19 cases, COVID-19 deaths, vaccination, prediction, worldwide
1. Introduction
Coronavirus disease 2019 (COVID-19) is a complex respiratory illness caused by SARS-CoV-2, a novel coronavirus first reported in Wuhan, China. COVID-19 is thus far the most severe pandemic of the 21st century, causing 176 million reported cases and 3.81 million deaths worldwide as of June 2021. SARS-CoV-2 has spread rapidly, overwhelming health systems and clinical management, and has been considered as an unprecedented public health challenge [1,2,3,4,5]. Therefore, strong preventive and control measures must be taken, such as social distancing, lockdown, wearing masks and maintaining a healthy lifestyle. Despite the actions taken, SARS-CoV-2 has had a profound impact on infected individuals as well as the entire population, negatively affecting the global economy and resources and leading to mental disorders, such as depression and anxiety [6,7]. Previous studies have shown that vaccine, as a powerful weapon to control an infectious disease, can notably allay or defeat diseases caused by pathogens [8,9]. There is an urgent need to develop vaccines to limit the spread of SARS-CoV-2 with the aim of achieving “herd immunity”.
Currently, several vaccines are in phase IV clinical trials, Pfizer/BioNTech, Moderna, AstraZeneca, Sinovac; and more than 200 vaccine candidates are also in their early clinical trials. Compared to previous vaccines, COVID-19 vaccine development has proceeded at an incredible pace and was quickly put into use owing to the coordinated effort between the scientific, medical community and government agencies [10]. The worldwide efforts to develop a safe and effective COVID-19 vaccine is extraordinary, and the vaccination research is now underway in many countries. However, due to the limited vaccine production capacity, the global supply of vaccines is in short condition. Moreover, with uneven levels of medical care and economic development across countries, there is a global imbalance distribution in vaccination [11].
Most studies on vaccine efficacy are clinical trial studies, which have strict conditions, and the calculated efficacy is only theoretical [12,13,14,15,16,17]. The most intuitive manifestation of vaccine effectiveness in the real world is the effect on the number of new cases and deaths of COVID-19, but no study has yet examined the association between COVID-19 vaccination and the numbers of new cases and deaths.
To address this knowledge gap, this study first provides comprehensive analyses of global vaccine delivery and then, after adjusting for multiple covariates, investigates the relationship between daily vaccination and the numbers of new cases and deaths per day to identify the effectiveness of the vaccination.
2. Material and Methods
2.1. Data Collection
2.1.1. Vaccination Data
Vaccination data were derived from the Our World in Data website [18], a vaccination dataset that uses the most recent official numbers from government health ministries in each country worldwide. The beginning time point was selected based on the date of the first country starting its vaccinations, and the end time point was the date of completion of our analysis, i.e., vaccination data were collected from 20 December 2020 to 25 April 2021. If a country was not included, it means that no one in that country was vaccinated or no vaccination data were published during the time frame of this study. The dataset contains 59 variables. A detailed explanation of each variable can be found at https://github.com/owid/covid-19-data/blob/master/public/data/owid-covid-codebook.csv (accessed on 25 October 2021). The variables used in this analysis were the daily total number of people who received at least one vaccine dose, daily total number of people who received all doses prescribed by the vaccination protocol, daily new confirmed cases of COVID-19 and daily new deaths attributed to COVID-19. We used a snippet to illustrate the main variables:
Three people take part in a vaccination program, to be given a vaccine that requires 2 doses to be effective against the disease. Dina has received two doses; Tommy has received one dose; Ellie has not received any dose. In our data, the total number of people receiving at least one dose of vaccine will be equal to 2 (Dina, Tommy); The total number of people fully vaccinated will be equal to 1 (Dina). The total number of people not vaccinated will be equal to 0 (Ellie).
2.1.2. Confounding Factors
Some studies have shown that meteorological factors, such as temperature, can affect vaccine efficacy and the motivation of the population to become vaccinated. For example, the stability of the vaccine will decrease at tropical ambient temperatures [19]. Outside the temperature range of 2–8 °C, the activity of the vaccine is greatly reduced [20]. In Alabama, USA, multiple health departments delayed vaccinations due to extreme weather, which led to a decrease in the population’s motivation to become vaccinated [21]. To control these confounding factors, meteorological data were collected from the Global Surface Summary of the Day (GSOD) https://www.ncei.noaa.gov/access/metadata/landing-page/bin/iso?id=gov.noaa.ncdc:C00516 (accessed on 25 October 2021), which includes global data obtained from the National Oceanic and Atmospheric Administration (NOAA). The daily mean temperature (°C, TEMP), dew point temperature (°C, DEWP), air pressure (kPa, STP), wind speed (m/s, WDSP) and precipitation (mm, PRCP) were calculated based on 26,531 fixed meteorological monitors globally.
2.2. Statistical Analysis
First, we described the distribution of vaccines in the first top 10 and the last top 10 countries around the world, including the time of vaccine initiation and vaccination rates in each country using aggregated vaccination data.
Then, we used a generalized additive model (GAM) to explore the relationship between daily vaccinated people and daily confirmed COVID-19 cases or deaths. GAM is an extension of the generalized linear model and can be used in time series analysis, dealing with the complex nonlinear relationship of various variables using a smooth function [22,23,24]. We calculated the percentage change (%, PC) in the number of daily cases or deaths with per 10,000 people receiving at least one dose of vaccine or being fully vaccinated for each country. We used the coefficients of the GAM model to calculate the PC value. The calculation formula is below:
where PC indicates the percent change in the daily number of new cases or deaths, and refers to the coefficient of daily people receiving at least one dose of vaccine or people becoming fully vaccinated from the GAM model. is the unit increase in the number of daily people at least one dose vaccine or people fully vaccinated. In our current study, was set to 10,000 to obtain an appropriate effect value.
Next, we used a two-stage analytic protocol to explore whether vaccination can affect the daily number of new COVID-19 cases or deaths. In the first stage, we determined the parameters of the GAM model. In the second stage, a random effect meta model was used to pool the PC values from each country. We then reported the pooled effect value and the corresponding 95% confidence interval (CI) as the PC value in the daily number of cases or deaths per 10,000 people vaccinated or fully vaccinated. More specifically, we first selected the link function. Considering that all dependent variables are counting data, we chose the Poisson distribution as the link function of GAM. Second, we defined the degree of freedom (df) of the time trend. In the base model, only the time trend controlled with a restricted cubic spline was included, and we calculated the AIC (Akaike information criterion) value based on different df of the time trend. The df with the minimum AIC value was defined as the best df for the time trend. Third, we put other covariates into the model. In brief, holiday and day of the week were put into the model as factors to eliminate the holiday effect. Meteorological variables, such as TEMP, DEWP, STP, WDSP and PRCP, were included in the model using a restricted cubic spline with the best df to control for confounding factors. Fourth, independent variables (daily people receiving at least one dose of vaccine or fully vaccinated) were included in the model. The GAM model fitted in this study is shown as follows:
where is the expected number of daily cases or deaths at Day t; is the intercept; denotes the number of people receiving at least one dose of vaccine or fully vaccinated at Day t; s() refers to the restricted cubic spline; and TEMP, DEWP, STP, WDSP and PRCP denote daily temperature, dew of point, air pressure and precipitation, respectively. Dow is an indicator of the day of the week, and Holiday is an indicator of the holiday effect. All the numbers in the model represent the best df in the restricted cubic spline function.
Next, we used two different lag structures to explore the lag effect of vaccination on the daily number of new cases or deaths. A single lag is expressed as lag1, lag2, …, lag30, where lag1 indicates that the daily number of cases or deaths in the current day is affected by the number of vaccine people in the previous day. Cumulative lag is shown as lag01, lag02, …, lag30, where lag01 refers to the daily number of deaths in the current day being affected by the average number of vaccinated people in the previous day and the current day. Considering that the vaccine becomes effective 3–4 weeks after injection [16], we chose a maximum lag period of 30 days.
Finally, in addition to building the GAM model using data from 187 countries worldwide, we also fit the GAM model using data from the United States alone. Since the daily number of new cases and deaths in the United States was maintained at a certain level during the study period (the average numbers of daily new cases and deaths were 112,679 and 1977, respectively), and the daily number of vaccinated people was also large (the average numbers of fully vaccinated people and people with at least one dose of vaccine were 917,360 and 1,104,708, respectively), the fitted model was highly reliable, and its results can provide a reference for vaccine distribution and administration in other countries.
In this study, R-4.0.3 software was used for basic statistical description and data cleaning. The “MGCV” and “Meta” packages in R software were used to fit the GAM model. A two-sided test was used in all statistical analyses, and the test level was α = 0.05. p < 0.05 was considered statistically significant.
The study protocol was approved by the Institutional Review Board at the School of Public Health, Capital Medical University. Written informed consent was not required because we used aggregated data and no individualized data.
2.3. Data Availability
The data that support the findings of this study are available at https://ourworldindata.org/covid-vaccinations and https://www.ncei.noaa.gov/access/metadata/landing-page/bin/iso?id=gov.noaa.ncdc:C00516 (accessed on 25 October 2021).
3. Results
3.1. Global Vaccination Overview
In total, 187 countries and regions were included in the study, and the study time ranged from 20 December 2020 to 25 April 2021. During the study period, 1,011,918,763 doses of vaccine were administered, 540,623,907 people received at least one dose of vaccine, and 230,501,824 people received two doses (see Supplementary: original country data.xlsx). In this study, the first two countries to begin vaccination were the United States and Israel on 20 December 2020, and the last five countries to begin vaccination were Djibouti, Libya, Lesotho, Niger and Somalia on 17 April 2021 (see Table 1 and Table S1).
Table 1.
The first and last top 10 countries of percentage of vaccine doses worldwide.
| Country Abbr. | Country Name | Continent | Total Vaccinations | Proportion (%) | |
|---|---|---|---|---|---|
| First top 10 | USA | United States | North America | 225,640,460 | 22.30 |
| CHN | China | Asia | 220,309,000 | 21.78 | |
| IND | India | Asia | 138,379,832 | 13.67 | |
| GBR | United Kingdom | Europe | 45,580,400 | 4.50 | |
| BRA | Brazil | South America | 37,730,651 | 3.72 | |
| DEU | Germany | Europe | 24,821,527 | 2.45 | |
| TUR | Turkey | Asia | 21,068,403 | 2.08 | |
| FRA | France | Europe | 19,225,460 | 1.90 | |
| IDN | Indonesia | Asia | 18,322,578 | 1.81 | |
| RUS | Russia | Europe | 18,080,498 | 1.79 | |
| Last top 10 | NRU | Nauru | Oceania | 168 | 1.66 × 10−5 |
| CMR | Cameroon | Africa | 400 | 4.00 × 10−5 | |
| TON | Tonga | Oceania | 500 | 4.94 × 10−5 | |
| ARM | Armenia | Asia | 565 | 5.58 × 10−5 | |
| LBY | Libya | Africa | 750 | 7.41 × 10−5 | |
| SSD | South Sudan | Africa | 947 | 9.36 × 10−5 | |
| PNG | Papua New Guinea | Oceania | 1081 | 1.07 × 10−4 | |
| NER | Niger | Africa | 1366 | 1.35 × 10−4 | |
| MSR | Montserrat | North America | 1751 | 1.73 × 10−4 | |
| SLB | Solomon Islands | Oceania | 2000 | 1.98 × 10−4 |
Abbr.: ISO 3166-1 alpha-3-three-letter country codes.
In this study, the top 10 countries with the highest total number of vaccines administered cumulatively accounted for 76.06% of the total number of vaccines administered globally. The country with the highest number of vaccines injected was the United States (225,640,460), accounting for 22.30% of the total number of vaccines worldwide, followed by China (220,309,000) and India (138,379,832), which accounted for 21.78% and 13.67% of the total number of vaccines administered worldwide, respectively, while the fourth place, the United Kingdom (45,580,400), accounted for only 4.50%.
The 10 countries with the lowest number of vaccines were mostly developing countries in Africa and Oceania, with a cumulative percentage of 0.94 per 100,000, indicating a highly uneven distribution of vaccines globally (see Table 1).
In the current study, some controversial regions (e.g., Gibraltar and Falkland Islands) were counted as individual vaccine injections, which resulted in some areas with smaller populations having a higher proportion of fully vaccinated population; thus, we screened countries or regions with a population size greater than 100,000 for statistical description (see Table S2).
In terms of the proportion of the fully vaccinated population relative to the total population, Israel ranked in first place, with 57.86% of the total population fully vaccinated. The second-ranked country was the United Arab Emirates with 38.79%, followed by Chile with 32.24%. Although the vaccination rate in the United States ranked the fifth among the top 10 countries, the total population in the US is 38.24 times larger than that in Israel, which ranked the first. It is a remarkable achievement for the United States to have fully vaccinated 28.12% of the total population. After screening other countries with the similar population size as the United States (100 million or more people, excluding China because there are no data on the fully vaccinated population), we can see that the ratio of the fully vaccinated people to the total population in Brazil, which ranked the second, is only 5.20%, while the ratio in the United States is 5.4 times higher than that in Brazil (see Table S3).
All 10 countries with the lowest proportion of fully vaccinated people to the total national population had a proportion of less than 1%, and most of them were in Asia and Africa.
3.1.1. The Association between Vaccination and Daily Cases and Deaths for COVID-19 Globally
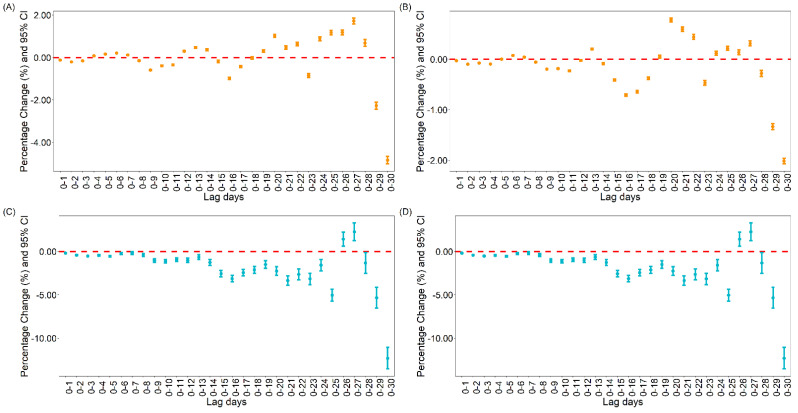
The GAM results showed a significant lag effect between vaccination and COVID-19 daily new cases or deaths both under the single lag model and moving lag model (see Figure 1 and Figure S1).
Figure 1.
Percentage change of daily cases and deaths with moving lag globally. (A) Percentage change of daily cases with daily 10,000 people fully vaccinated increasing; (B) Percentage change of daily cases with daily 10,000 new people vaccinated increasing; (C) Percentage change of daily deaths with daily 10,000 people fully vaccinated increasing; (D) Percentage change of daily deaths with daily 10,000 people vaccinated increasing.
For the relationship between vaccination and new COVID-19 cases, the results showed that the daily new cases of COVID-19 would be reduced by 24.43% [95% CI: 18.89, 29.59] and 7.50% [95% CI: 6.18, 8.80] with 10,000 people per day becoming fully vaccinated and 10,000 people per day with at least one dose of vaccine at lag0–30 and lag0–29 under the moving lag model, respectively.
For the relationship between vaccination and new COVID-19 deaths, the results showed that the daily new deaths of COVID-19 would be reduced by 13.32% [95% CI: 3.81, 21.89] and 2.02% [95% CI: 0.18, 4.16] with 10,000 people per day becoming fully vaccinated and 10,000 people per day with at least one dose of vaccine both at lag0–30 under moving lag model, respectively. Similar results were also found under the single lag model (see Figure S1).
3.1.2. The Association between Vaccination and Daily Cases and Deaths for COVID-19 in the United States
For the relationship between vaccination and new COVID-19 cases in the United States, the results showed that 10,000 fully vaccinated people per day and 10,000 people per day with at least one dose of vaccine would reduce the new COVID-19 cases by 4.84% [95% CI: 4.66, 5.02] and 2.02% [95% CI: 1.96, 2.07] both at lag0–30 under the moving lag GAM model, respectively (see Figure 2).
Figure 2.
Percentage change in daily cases and deaths with moving lag in the US. (A) Percentage change of daily cases with daily 10,000 people fully vaccinated increasing; (B) Percentage change of daily cases with daily 10,000 people vaccinated increasing; (C) Percentage change of daily deaths with daily 10,000 people fully vaccinated increasing; (D) Percentage change of daily deaths with daily 10,000 people vaccinated increasing.
For the relationship between vaccination and new COVID-19 deaths in the United States, the results showed that 10,000 fully vaccinated people per day and 10,000 people per day with at least one dose of vaccine would reduce the new COVID-19 deaths by 12.31% [95% CI: 11.06, 13.53] and 3.36% [95% CI: 2.97, 3.76], respectively, at lag0–30 under the moving lag GAM model. Similar results were found under the single lag model (see Figure S3).
3.1.3. Daily Cases and Deaths Predicted for COVID-19 with 10,000 Fully Vaccinated People
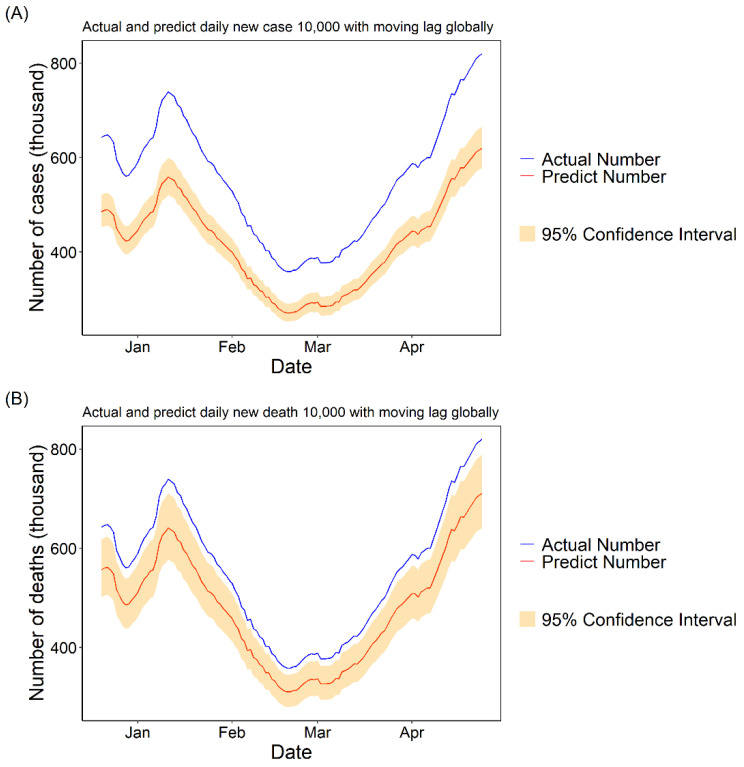
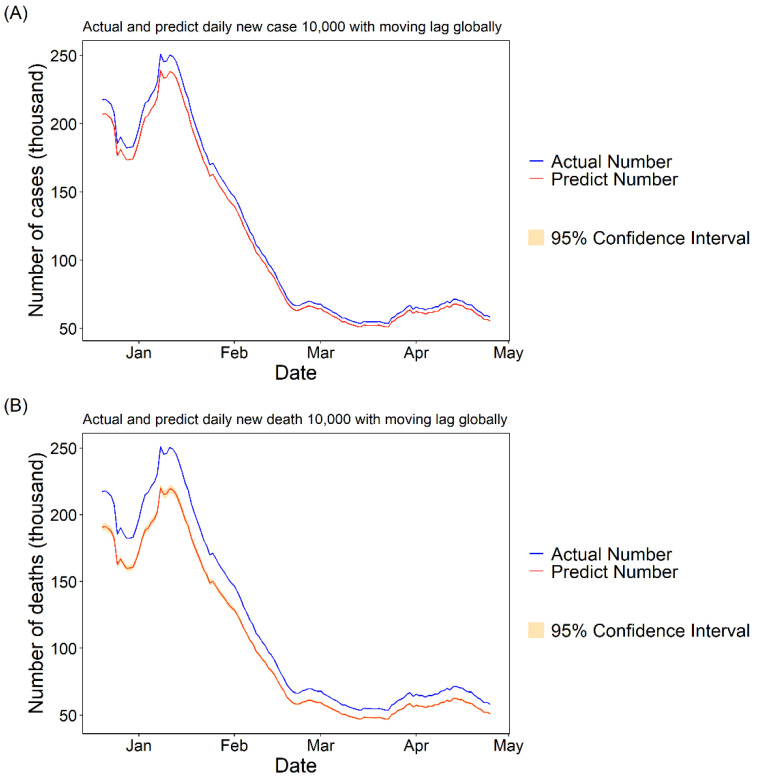
To visualize the results of the GAM model intuitively, we used the PC values calculated from the moving lag GAM model multiplied by the true daily cases and deaths of COVID-19 to show the theoretical daily cases and deaths of COVID-19 if 10,000 people had received two doses of vaccine per day during the study period (we considered those who received two doses of vaccine to be fully immunized). The results showed that there would be a significant decrease in new COVID-19 cases and deaths per day both in the United States and globally (see Figure 3 and Figure 4).
Figure 3.
The actual and predicted daily cases and deaths numbers with a moving lag globally. The predicted number was calculated by multiplying the actual number of daily cases by the PC value of the best lag. (A) The PC value and related 95% CI are −24.43 [−29.59, −18.89] with the best moving lag of 30. (B) The PC value and related 95% CI are −13.32 [−21.89, −3.81] with the best moving lag of 30.
Figure 4.
The actual and predicted daily cases and deaths numbers in the US. The predicted number was calculated by multiplying the actual number of daily cases by the PC value of the best lag. (A) The PC value and related 95% CI are −4.84 [−5.02, −4.66] with the best moving lag of 30. (B) The PC value and related 95% CI are −12.31 [−13.53, −11.06] with the best moving lag of 30.
4. Discussion
Our study began with the describing vaccination in 187 countries and regions worldwide, including people who received at least one dose of vaccine and two doses of vaccine (fully vaccinated people). Then, we used the GAM model to explore the relationship between daily vaccinated people and COVID-19 cases and deaths both globally and in the USA. Finally, we used the PC values calculated from the moving lag GAM model to predict the theoretical daily cases and deaths of COVID-19 if 10,000 people had received two doses of vaccine per day during the study period.
We found that vaccines were very unevenly distributed across countries globally. The top 10 countries with the highest total vaccine injections cumulatively accounted for 76.06% of the total global vaccine injections, while the bottom 10 countries accounted for less than 1% of total vaccine injections. As a result, some countries have distributed the second dose of vaccine to those who have never been vaccinated in order to deliver the first vaccination to as many people as possible. The practice of devoting a limited amount of first-dose vaccine to a larger number of people reduces the infection rate of COVID-19 to a certain extent, but in the long run, delivering only a single dose of vaccine may promote virus mutations [25]. The rate at which a virus mutates is closely related to the human body’s immune response. The less stress the immune response gives, the faster the virus adapts. If the immunity of some individuals is insufficient to inhibit viral replication, it may induce a variant with “immune escape” potential [25].
Harvard predicted that the human pandemic of COVID-19 could not end in a short term [26]. Vaccination has played a central role in the COVID-19 pandemic, especially in stopping new infections and saving millions of lives [14,27,28,29,30]. In contrast with single-dose vaccines, all approved coronavirus vaccine shots need to be injected as two doses, spaced apart. While the first dose helps build a sufficient immune response in the body, the second dose helps strengthen it and generates memory B cells that ‘remember’ the response. Thus, only after a person receives two full doses of the vaccine in a given timeline, is he or she considered to be fully vaccinated and protected. For economically developed countries, the vaccination rate is relatively high, especially for countries such as the United States, where the proportion of the population receiving two doses of vaccine is 5.4 times higher than that of the second-place country, Israel. Pandemic prevention and control are not a national or a regional effort; once a supervirus emerges during the spread of a pandemic, all humanity will be affected. To effectively contain the spread of COVID-19 globally, large economically developed countries need to help small countries so that we can end the epidemic as soon as possible.
Our study confirmed that the COVID-19 vaccine continues to work well in the real world, except in rigorous clinical trials, filling a gap in relevant research [17,27,30,31]. We found that with every increase of 10,000 fully vaccinated people, new cases would be reduced by 24.43% [95% CI: 18.89, 29.59] and new deaths would be reduced by 13.32% [95% CI: 3.81, 21.89] per day. The prediction curves also show that if 10,000 people per day received two doses of the vaccine, there would be a significant reduction in the daily number of new COVID-19 cases and deaths per day. Thanks to the high efficiency of the various vaccines, vaccination was able to significantly reduce the number of daily COVID-19 cases and deaths. The minimum effectiveness of the COVID-19 vaccine has been defined as 50% after discussions among WHO experts [32], and the currently marketed vaccines have higher than expected effectiveness of 95% for the Pfizer-BioNTech vaccine [16], 62.1% for Oxford-AstraZeneca [33], 94.1% for Moderna [34] and 91.6% for Gamaleya [35], among others.
Although we did not have access to the specific type of vaccines given to each individual in the current study, we still believe that our results have a high level of reliability. Inactivated virus vaccines, adenovirus-based vaccines and mRNA-based vaccines are the main available vaccines, but their immune response mechanisms are different [36]. Currently, vaccination is administered globally by giving the same type of vaccine, but some scholars are also exploring novel vaccination strategies, such as combining different types of COVID-19 vaccines, also known as heterologous vaccination, by which the vaccinated population may achieve better immunogenicity [37]. The mechanism of action has been elucidated through relevant studies, showing that heterologous vaccination enhances cytotoxic T-cell and Th1-activated immune responses [38]. Therefore, a clinical trial called ComCOV comparing combinations of COVID-19 vaccine schedules is underway to assess whether there are differences in the effectiveness of heterologous and homologous vaccination with COVID-19 vaccines [39]. These results will shed light on possible approaches regarding COVID-19 vaccination.
Our study has some strengths. First, we included 187 countries and regions worldwide, and the large scope of the study allowed us to provide a more comprehensive picture of the relationship between daily vaccinations and COVID-19 cases and deaths. Then, we used a two-stage analytic protocol method that gave a more accurate result. The GAM model used in stage one could deal with the complex nonlinear relationship of various variables in a time series analysis. The random effect meta model used in stage two aggregated the effect values for each country according to different weights. There are also limitations that need to be addressed. First, the study period was 128 days from 20 December 2020, to 25 April 2021, and longer continuing vaccination monitoring would enable collection of a more stable time series. Third, due to the lack of age- or sex-stratified datasets, we could not observe more significant results in more detailed stratifications at the population level.
5. Conclusions
In summary, for the first time, the results of our analysis show that vaccination can effectively reduce the new cases and deaths of COVID-19 in the real world, but vaccines are not evenly distributed across countries. Our study provides a theoretical basis for global COVID-19 prevention and control efforts; that is, we must accelerate the speed of vaccination and promote its fair distribution worldwide to eliminate the COVID-19 pandemic as quickly as possible.
Acknowledgments
The data were obtained from the OWID (Our World in Data). We thank all of the health care personnel who contributed to the detection, epidemiological investigation, and diagnosis of the COVID-19 vaccine.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9111328/s1, Table S1: The first and last top 10 countries to start vaccinating, Table S2: Top 10 countries in proportion of fully vaccinated with population more than 100,000, Table S3: Proportion of fully vaccinated with population more than 100,000,000, Table S4: Top 20 countries and regions in proportion of fully vaccinated, Figure S1: Percentage change of daily cases and deaths with single lag globally, Figure S2: The actual and predict* daily cases and deaths number with single lag globally, Figure S3: Percentage change of daily cases and deaths with single lag in US, Figure S4: The actual and predict* daily cases and deaths number in US.
Author Contributions
Z.L.: Writing—review and editing, data collection and cleaning, methodology, software. X.L. (Xiangtong Liu): writing—review and editing, supervision. M.L. (Mengyang Liu): writing original draft, data collection and cleaning, methodology, visualization. Z.W.: data collection, methodology, visualization. Y.L. (Yue Liu): visualization, methodology, data collection, methodology. W.L.: writing—review and editing. M.L. (Mengmeng Liu): methodology. X.W.: writing—review and editing, supervision. B.G.: data collection and cleaning. Y.L. (Yanxia Luo): writing—review, methodology. X.L. (Xia Li): writing—review and editing, supervision. L.T.: writing—review and editing, conceptualization, project administration, supervision. W.W.: writing—review and editing, supervision. X.G.: writing—review and editing, conceptualization, project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Program of the Natural Science Fund of China (No. 82003559). The sponsors of the study had no role in the study design, data collection, data analysis, interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://ourworldindata.org/covid-vaccinations (accessed on 25 October 2021).
Conflicts of Interest
We declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barco A.A.D., Ortega M.A. Epidemiology and public health in the COVID-19 epidemic. Medicine. 2020;13:1297–1304. doi: 10.1016/j.med.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamadian M., Chiti H., Shoghli A., Biglari S., Parsamanesh N., Esmaeilzadeh A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021;23:e3303. doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumder J., Minko T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021;23:14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dao T.L., Hoang V.T., Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: A narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:13–25. doi: 10.1007/s10096-020-04088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habas K., Nganwuchu C., Shahzad F., Gopalan R., Haque M., Rahman S., Majumder A.A., Nasim T. Resolution of coronavirus disease 2019 (COVID-19) Expert Rev. Anti-Infect. Ther. 2020;18:1201–1211. doi: 10.1080/14787210.2020.1797487. [DOI] [PubMed] [Google Scholar]
- 6.Choi E.P.H., Hui B.P.H., Wan E.Y.F. Depression and Anxiety in Hong Kong during COVID-19. Int. J. Environ. Res. Public Health. 2020;17:3740. doi: 10.3390/ijerph17103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talevi D., Socci V., Carai M., Carnaghi G., Faleri S., Trebbi E., di Bernardo A., Capelli F., Pacitti F. Mental health outcomes of the COVID-19 pandemic. Riv. Psichiatr. 2020;55:137–144. doi: 10.1708/3382.33569. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisse M., Vrba S.M., Kirk N., Liang Y., Ly H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020;11:583077. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 11.Pollard C.A., Morran M.P., Nestor-Kalinoski A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020;52:549–557. doi: 10.1152/physiolgenomics.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., Wolfson A.R., Williams P., Khan D.A., Phillips E., et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., Santos M.R., Schuitemaker H., Watson M., Arvin A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020;12:eabe0948. doi: 10.1126/scitranslmed.abe0948. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021;21:e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrotra D.V., Janes H.E., Fleming T.R., Annunziato P.W., Neuzil K.M., Carpp L.N., Benkeser D., Brown E.R., Carone M., Cho I., et al. Clinical Endpoints for Evaluating Efficacy in COVID-19 Vaccine Trials. Ann. Intern. Med. 2021;174:221–228. doi: 10.7326/M20-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coronavirus (COVID-19) Vaccinations. [(accessed on 12 November 2021)]. Available online: https://ourworldindata.org/covid-vaccinations.
- 19.Kis Z., Shattock R., Shah N., Kontoravdi C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019;14:e1800376. doi: 10.1002/biot.201970055. [DOI] [PubMed] [Google Scholar]
- 20.Tebas P., Kraynyak K.A., Patel A., Maslow J.N., Morrow M.P., Sylvester A.J., Knoblock D., Gillespie E., Amante D., Racine T., et al. Intradermal SynCon® Ebola GP DNA Vaccine Is Temperature Stable and Safely Demonstrates Cellular and Humoral Immunogenicity Advantages in Healthy Volunteers. J. Infect. Dis. 2019;220:400–410. doi: 10.1093/infdis/jiz132. [DOI] [PubMed] [Google Scholar]
- 21.Here’s How Severe Weather Is Affecting COVID-19 Vaccinations in These States. [(accessed on 26 October 2021)]. Available online: https://www.cnn.com/2021/02/18/us/states-vaccine-delays-winter-weather/index.html.
- 22.Qi H., Xiao S., Shi R., Ward M.P., Chen Y., Tu W., Su Q., Wang W., Wang X., Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci. Total Environ. 2020;728:138778. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trevor Hastie R.T. Generalized Additive Models. Chapman & Hall/CRC; Boca Raton, FL, USA: 1990. [Google Scholar]
- 24.Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saad-Roy C.M., Morris S.E., Metcalf C.J.E., Mina M.J., Baker R.E., Farrar J., Holmes E.C., Pybus O.G., Graham A.L., Levin S.A., et al. Epidemiological and evolutionary considerations of SARS-CoV-2 vaccine dosing regimes. Science. 2021;372:363–370. doi: 10.1126/science.abg8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telenti A., Arvin A., Corey L., Corti D., Diamond M.S., García-Sastre A., Garry R.F., Holmes E.C., Pang P.S., Virgin H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.-D., Chi W.-Y., Su J.-H., Ferrall L., Hung C.-F., Wu T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020;27:104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soleimanpour S., Yaghoubi A. COVID-19 vaccine: Where are we now and where should we go? Expert Rev. Vaccines. 2021;20:23–44. doi: 10.1080/14760584.2021.1875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marian A.J. Current state of vaccine development and targeted therapies for COVID-19: Impact of basic science discoveries. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2021;50:107278. doi: 10.1016/j.carpath.2020.107278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funk C.D., Laferrière C., Ardakani A. Target Product Profile Analysis of COVID-19 Vaccines in Phase III Clinical Trials and Beyond: An Early 2021 Perspective. Viruses. 2021;13:418. doi: 10.3390/v13030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Montero C., Fraile-Martínez O., Bravo C., Torres-Carranza D., Sanchez-Trujillo L., Gómez-Lahoz A.M., Guijarro L.G., García-Honduvilla N., Asúnsolo A., Bujan J., et al. An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times. Vaccines. 2021;9:433. doi: 10.3390/vaccines9050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer A.J., McKay P.F., Belij-Rammerstorfer S., Ulaszewska M., Bissett C.D., Hu K., Samnuan K., Blakney A.K., Wright D., Sharpe H.R., et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021;12:2893. doi: 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comparing COVID-19 Vaccine Schedule Combinations—Com-COV. [(accessed on 25 October 2021)]. Available online: https://www.comcovstudy.org.uk/about.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://ourworldindata.org/covid-vaccinations (accessed on 25 October 2021).