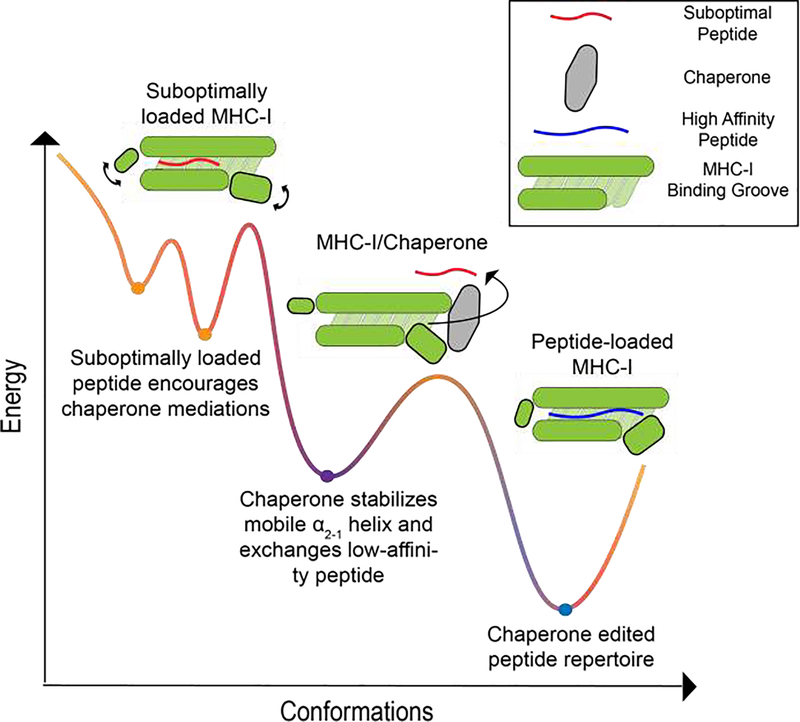
Figure 3.
A conceptual energy landscape depicting the progressive loading of an MHC-I molecule with a high-affinity peptide. MHC-I molecules can bind to low-affinity peptides forming a suboptimal-loaded complex that is unstable and conformationally heterogeneous. Molecular chaperones, like TAPBPR, can catalyze low-affinity peptide dissociation by widening the groove. Ultimately, the binding of a high-affinity peptide quenches MHC-I dynamics, stabilizes the MHC-I molecule and displaces TAPBPR.