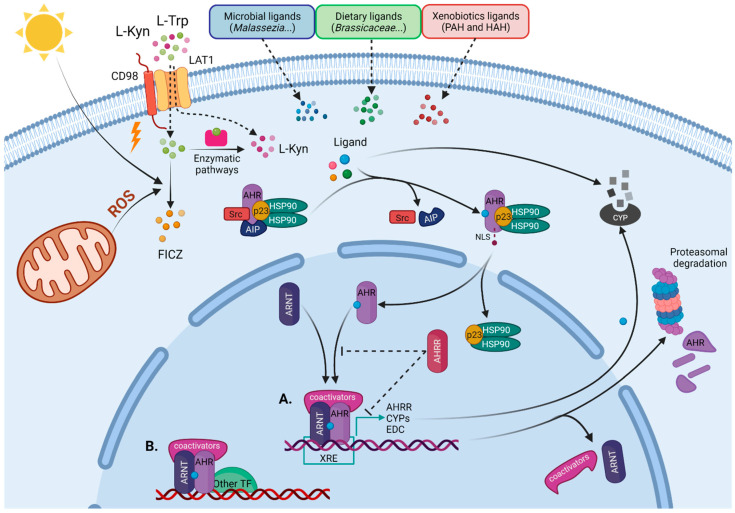
Figure 1.
AHR genomic signaling pathway. Before ligand binding, AHR is bound by a chaperone complex (described in the text and the figure), which maintains its localization in the cytoplasm. Cells are exposed to different AHR ligands, such as bioproducts of microbiota, phytochemicals, xenobiotics, or endogenous ligands, mostly derived from L-tryptophan (L-Trp). When a ligand binds, the AHR changes its conformation and c-Src and AHR-interacting proteins (AIP) are released, resulting in the exposure of the nuclear localization signal (NLS) in the AHR’s N-terminus, that allows docking of importin β and mediates nucleocytoplasmic shuttling. Once in the nucleus, the ligand-activated AHR heterodimerizes with its protein partner, the AHR nuclear translocator (ARNT), at the time it dissociates of cytoplasmic chaperone complex. (A) The ligand–AHR–ARNT heterodimeric complex binds specific DNA sequences located in the promoter regions of target genes, named xenobiotic responsive elements (XRE), and recruits additional coactivators and components of the transcriptional machinery (described in the text) that are required to initiate transcription of the target gene. (B) The ligand–AHR–ARNT complex can also interact with non-canonical AHR partners and regulate additional target genes. (A) Canonical genes include enzymes of the cytochrome P450 (CyP) family and AHR repressor (AHRR). CyP enzymes metabolize AHR ligands and the AHRR competes with the AHR for interaction with the ARNT and DNA binding. After transcription, the AHR is exported out of the nucleus and is rapidly degraded by the proteasome. PAH—polycyclic aromatic hydrocarbon; HAH—halogenated aromatic hydrocarbon; ROS—reactive oxygen species; HSP90—90 kDa heat shock protein; EDC—epidermal differentiation complex; TF—transcriptional factor. Figure was created with BioRender.com.