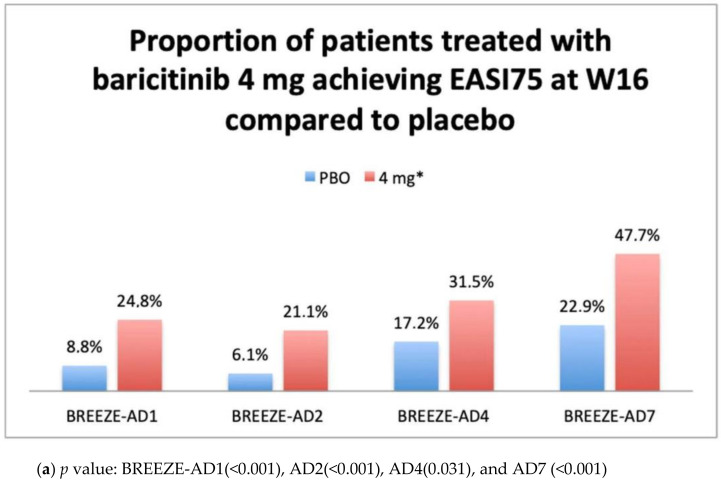
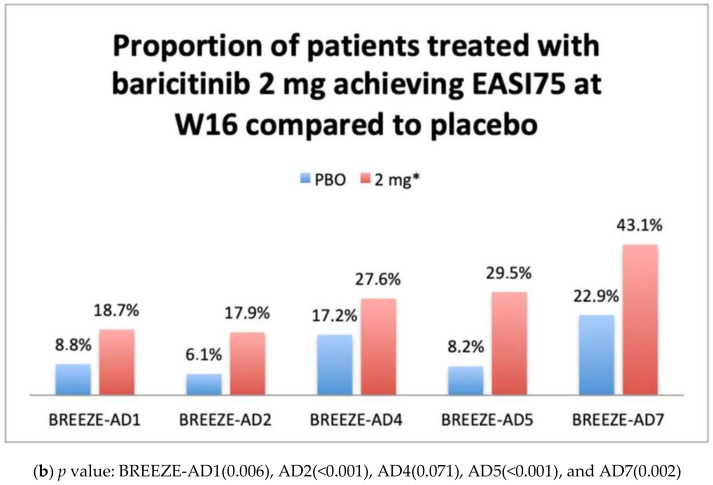
Figure 2.
(a) Primary efficacy endpoint from BREEZE AD1, AD2, AD4, and AD7: Proportions of patient treated with baricitinib 4 mg achieving EASI75 at W16 compared to placebo. Abbreviations: PBO, placebo; EASI, Eczema Area Severity Index; W16, week 16. * In BREEZE-AD 4 and AD7, baricitinib was administered in association with topical corticosteroid as for protocol. (b) Primary efficacy endpoint from BREEZE-AD1, AD2, AD4, AD5, and AD7: Proportions of patient treated with baricitinib 2 mg achieving EASI75 at W16 compared to placebo. Abbreviations: PBO, placebo; EASI, Eczema Area Severity Index; W16, week 16. * In BREEZE-AD 4 and AD7, baricitinib was administered in association with topical corticosteroid as for protocol.