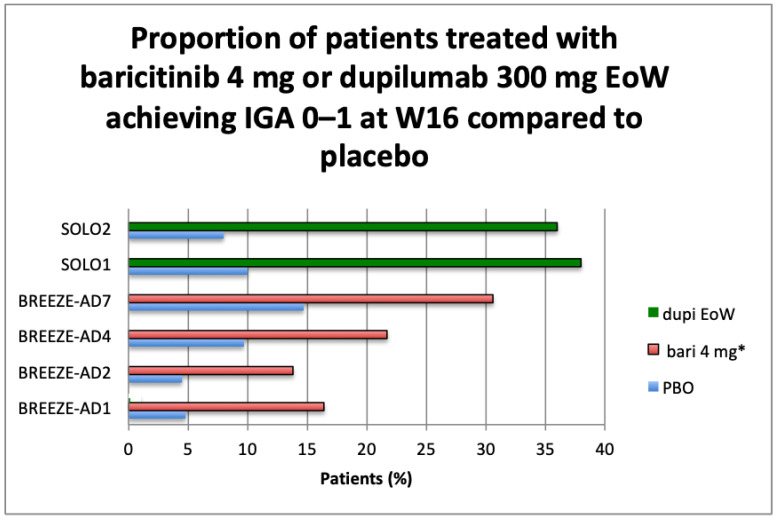
Figure 3.
Overview of primary efficacy endpoint from RCTs: Proportion of patients treated with baricitinib 4 mg or dupilumab 300 mg EoW achieving IGA 0–1 at W16 compared to placebo. Abbreviations: PBO, placebo; IGA, investigator global assessment; W16, week 16; EoW, every other week; Dupi, dupilumab; bari, baricitinib. * In BREEZE-AD 4 and AD7, baricitinib was administered in association with topical corticosteroid as for protocol.