Table 2.
Common names, IUPAC nomenclatures, molecular formulas and chemical structures of the most commercialized xanthophylls.
| Common Names | IUPAC Nomenclature | Molecular Formulas | Chemical Structures | References |
|---|---|---|---|---|
| Fucoxanthin | 3,5′-Dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate | C42H58O6 |
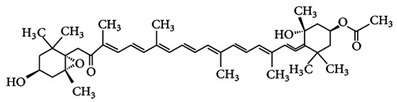 3S,5R,6S,3′S’,5′R,6′R)-3,5′-dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate |
[91] |
| ASX | 3,3′-Dihydroxy-β,β-carotene-4,4′-dione | C40H52O4 |
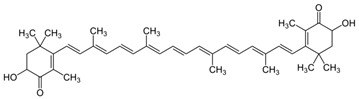 (3S,30S)-3,30-dihydroxy-β,β-carotene-4,40-dione |
[12] |
| Lutein | β- ε-Carotene-3,3′-diol | C40H56O2 |
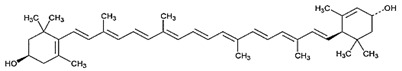 (3R,3′R,6′R)-β,ε-carotene-3,3′-diol |
[92] |
| Zeaxanthin | β,β-Carotene-3,3′-diol | C40H56O2 |
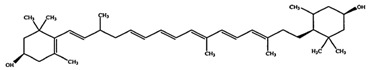 (3R,30R)-β,β-carotene-3,3′-diol |
[92] |
| Violaxanthin | 5,5′,6,6′-Tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3,3′-diol | C40H56O4 |
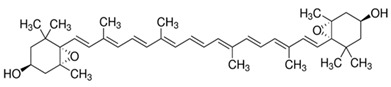 S,3′S,5R,5′R,6S,6′S)-5,5′,6,6′-tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3 |
[93] |
| Canthaxanthin | β,β-Carotene-4,40-dione | C40H52O2 |
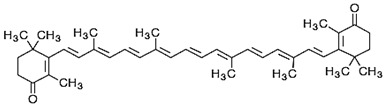 trans-β-carotene-4,4′-dione |
[94] |
| β-Cryptoxanthin | β,β-Caroten-3-ol | C40H56O |
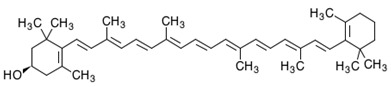 (3R)-β,β-Caroten-3-ol |
[95] |
| Diadinoxanthin | 5,6-Epoxy-7’,8’-didehydro-5,6-dihydro-b,b-carotene-3,3-diol | C40H54O3 |
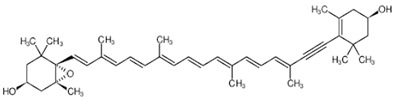 (3S,3’R,5R,6R)-7’,8’-Didehydro-3,6-epoxy-5,6-dihydro- β, β -carotene-3’,5-diol |
[61] |
| Diatoxanthin | 3,3′-7,8-Didehydro-ß,ß-carotene-3,3’-diol | C40H54O2 |
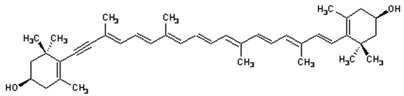 (3R,3’R)-7,8-Didehydro- β, β -carotene-. 3,3’-diol |
[61] |
