Table 1.
Characteristics of sorafenib, lenvatinib, and cabozantinib.
| Drug | Target | Half-Life in Plasma | Metabolism | Approval for DTC | Structure |
|---|---|---|---|---|---|
| Sorafenib | VEGFR-2 and -3 PDGFR, c-Kit, RET/PTC, RAF [41] | 36 h [41] | Hepatic CYP3A4 and UGT1A9 [42] | 2013 [43] |
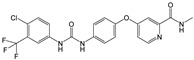
|
| Lenvatinib | , RET, c-KIT, FGFR 1-4 [3] | 28 h [44] | Hepatic CYP3A4 [45] | 2015 [28] |
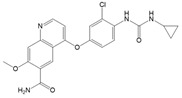
|
| Cabozantinib | VEGFR-2, c-MET, RET [31] | 100–120 h [46] | Hepatic CYP3A4 [46] | 2021 [47] |
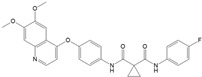
|
Structures were drawn with ChemDraw Professional 17 (Perkin Elmer Informatics, Waltham, MA, USA).
