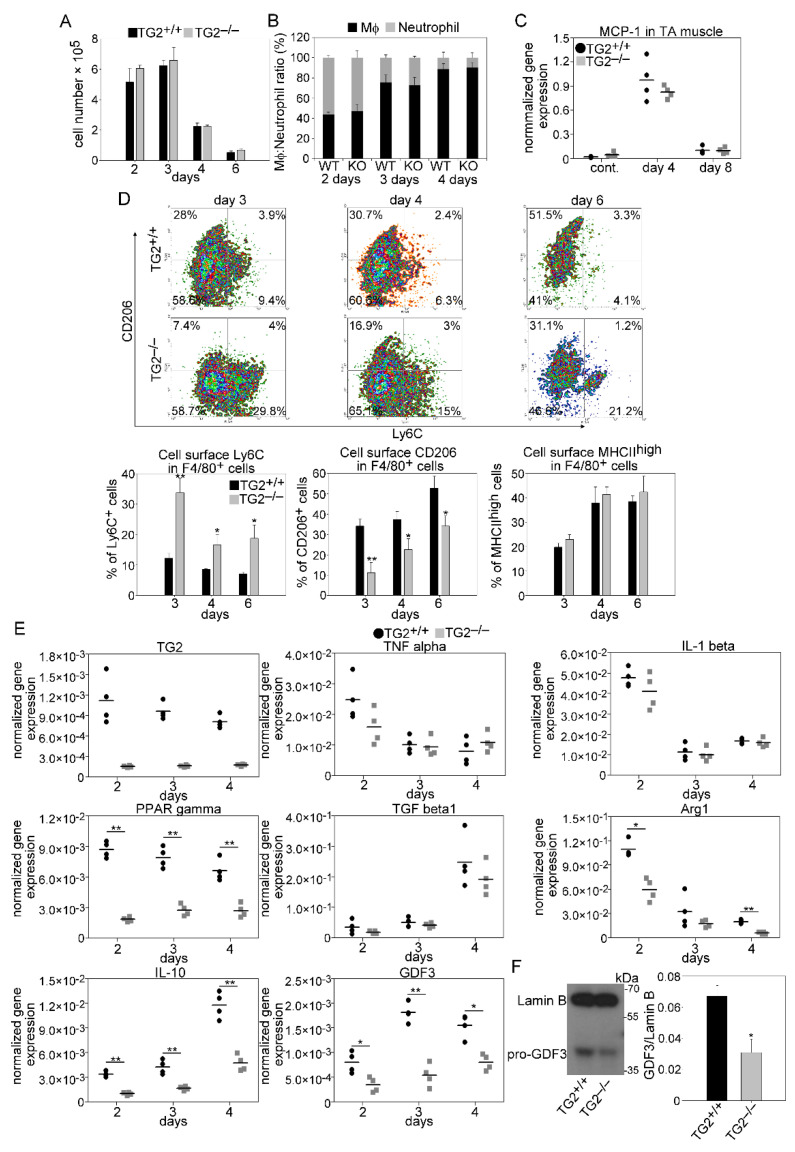
Figure 6.
As compared to wild-type mice, leukocyte infiltration is not altered in TG2 null regenerating TA muscles following CTX injury, but the pro-inflammatory to healing phenotypic conversion of macrophages is delayed. (A) Number of CD45+ leukocytes per injured muscle and (B) ratio of anti-F4/80 antibody stained Mϕs and anti-Ly6G/Ly6C (GR-1) stained neutrophils within the CD45+ leukocyte population in TG2+/+ and TG2−/− TA muscles during the first 4 days of regeneration following CTX-induced injury (n = 3). (C) MCP-1 mRNA expression levels of muscle-derived wild-type or TG2 null CD45+ leukocytes determined by qRT-PCR following CTX-induced injury (n = 4). (D) Representative scatter plots of CD206 and Ly6C stained muscle-derived F4/80+ cells and the percent of Ly6C+, CD206+, and MHCIIhigh cells within the muscle-derived F4/80+ population determined at the indicated days following CTX-induced injury in the TA muscles of TG2+/+ and TG2−/− mice (n = 3). (E) mRNA expressions of TG2 and pro-and anti-inflammatory marker genes in CD45+ cells isolated from TA muscles determined by qRT-PCR at day 2, 3, and 4 post-injury (n = 4). (F) Protein level of GDF3 at day 4 post-injury in CD45+ cells isolated from regenerating TA muscles determined by Western blot analysis (n = 3). All data are expressed as mean ± SD, while dots represent data from individual animals. N in all these experiments corresponds to number of animals injured on both legs to harvest enough leukocytes from each mouse. Asterisks indicate statistically significant difference (* p < 0.05, ** p < 0.01, Student’s t-test).