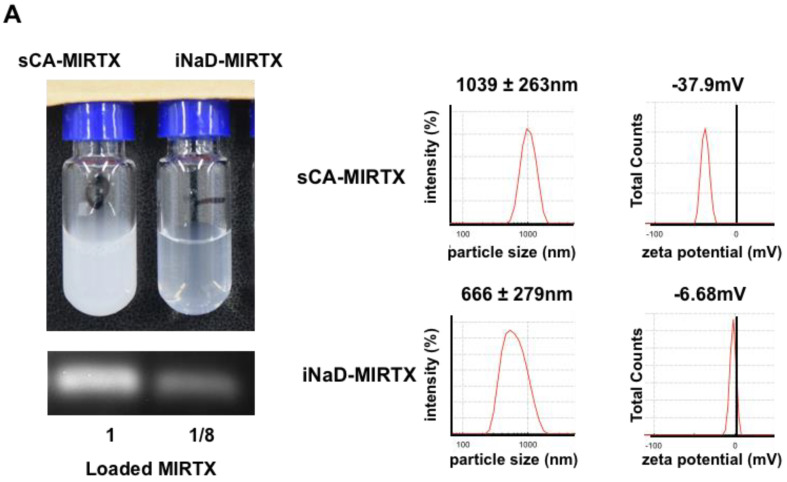
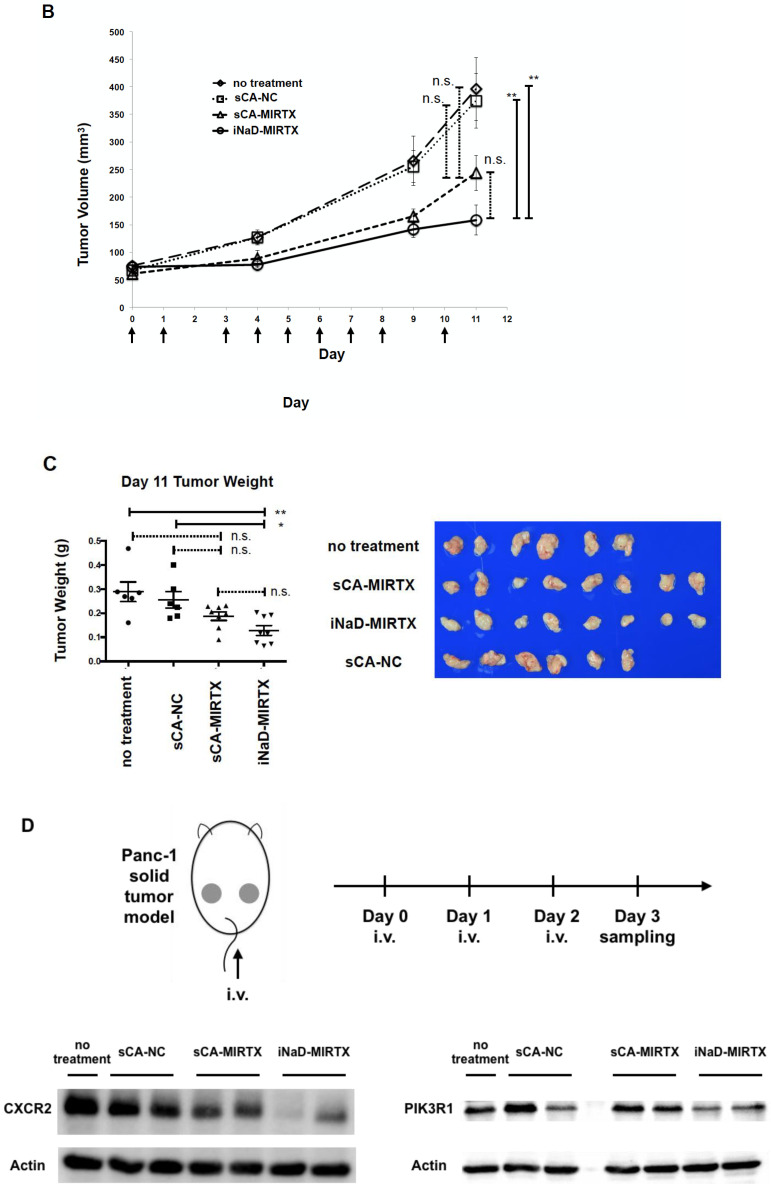
Figure 6.
Anti-tumor effect of iNaD-MIRTX. (A) sCA-MIRTX and iNaD-MIRTX were prepared for one intravenous injection, followed by measurement of loaded MIRTX, DLS, and zeta potential analysis. The MIRTX loading of sCA-MIRTX was 24 μg, and that of iNaD-MIRTX was 8 μg. (B) Therapeutic model of Panc-1 tumors. sCA-MIRTX (MIRTX loading: 24 μg/injection), sCA-NC (negative control miRNA loading: 24 μg/injection), or iNaD-MIRTX (MIRTX loading: 3 μg/injection) was intravenously administered on days 0, 1, 3, 4, 5, 6, 7, 8, and 10. Data represent mean ± SEM (n = 6–8 tumors from 3–4 mice). ** p < 0.01, n.s. = not significant, one-way ANOVA with Tukey’s multiple comparisons test. (C) Tumor weight on day 11. Data represent mean ± SEM (n = 6–8 tumors from 3–4 mice). * p < 0.05, ** p < 0.01, n.s. = not significant, one-way ANOVA with Tukey’s multiple comparisons test. (D) Mice were administered with sCA-MIRTX (MIRTX loading: 24 μg/injection) or iNaD-MIRTX (MIRTX loading: 3 μg/injection) on days 0, 1, and 2. Tumors were removed on day 3, and western blot analysis for CXCR2 and PIK3R1 was performed (n = 1–2 tumors from 2 mice for each group).