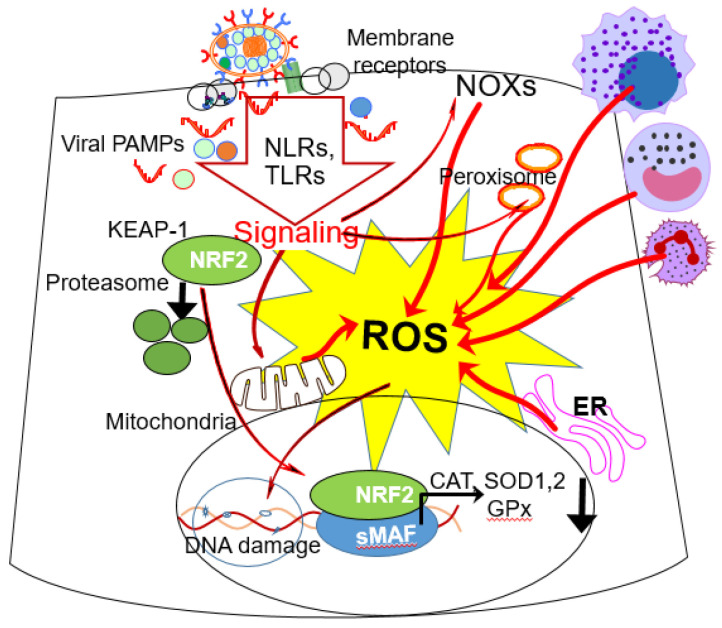
Figure 1.
Cellular sources of ROS in respiratory viral infections. Activation of receptors liganded by RSV, influenza and SARS-CoV-2 envelope proteins and the recognition of viral PAMPs by intracellular sensors (NLRs, TLRs) trigger signaling pathways, leading to activation of oxidoreductases located in cell membranes, endoplasmic reticulum, peroxisomes, and mitochondria. NADPH oxidases (NOXs) are the primary enzyme complexes in nearly all cell types, particularly in granulocytes and macrophages, along with oxidoreductases in mitochondrial complex I and II, which partially oxidize oxygen molecules to generate superoxide anion (O2•−). O2•− via Fenton and/or Haber-Weiss reactions are converted into hydroxyl radical (•OH). The highly reactive •OH reacts with proteins, lipids and DNA. ROS themselves, but particularly peroxidation of polyunsaturated fatty acids, trigger nuclear translocation of nuclear factor erythroid 2-related factor 2 (NRF2), which heterodimerizes with small musculoaponeurotic fibrosarcoma (MAF) transcription factor and binds the cis-acting enhancer antioxidant response element (ARE), leading to the expression of antioxidant enzymes, including Cu/Zn-superoxide dismutase (SOD1), glutathione peroxidases (GPXs) and catalase (CAT). However, in RSV and SARS-CoV-2infected cells and lungs there is a progressive decrease in levels of NRF2 via increased protein ubiquitination and its degradation through a proteasomal pathway [93,99,100,101]. Although ROS generation in RSV, SARS-CoV-2 infected cells is similar, it seems that NRF2 primarily modifies influenza A entry and replication [102]. In addition to the above-described pathways, activated monocytes and polymorphonuclear cells, in particular, neutrophils, have been shown to produce ROS. Abbreviations: NLR, nucleotide-binding oligomerization domain-like receptors; TLR, TOLL-like receptors, PAMP, pathogen-associated molecular patterns; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; ER, endoplasmic reticulum.