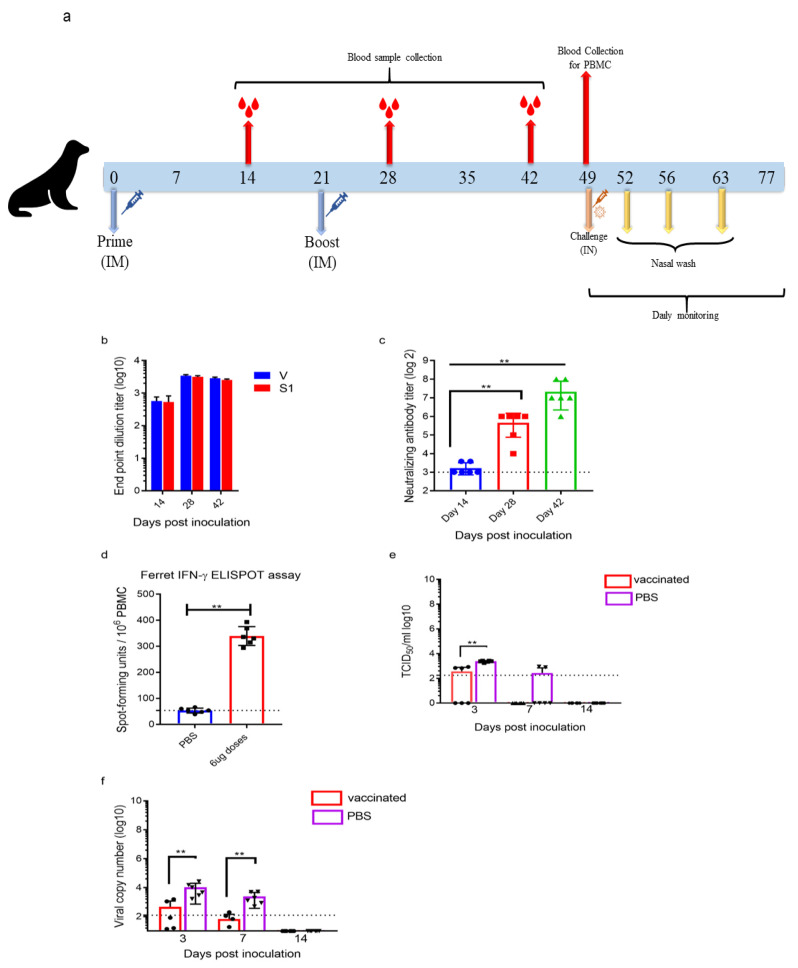
Figure 5.
Immune responses and protective efficacy of ERUCoV-VAC in ferrets. (a) Scheme of sample collection, immunization regimens, and SARS CoV-2 challenge. Groups of ferrets (n = 6) immunized on days 0 and 21 with 6 µg of ERUCoV-VAC or with a PBS (n = 6) via the intramuscular route. The ferrets were challenged 4 weeks after the second immunization with 5 × 105 TCID50 of SARS-CoV-2 by the intranasal route. (b) Serum IgG titres were detected by SARS CoV-2-specific S1-RBD (S1) and virion (V) ELISA in ferrets vaccinated with a 6 μg dose of ERUCoV-VAC. (c) Serum Nab titres determined by microneutralizing assay in ferrets vaccinated with a 6 μg dose of ERUCoV-VAC. (d) Cellular immune responses in the 6 μg dose group (n = 6) and control group (n = 6) were analysed on day 28 following the second immunization by an IFN-γ-based ELISPOT assay. On day 28 after the second immunization, a total of 12 ferrets, both vaccinated (n = 6) and unvaccinated (n = 6), were intranasally challenged with 5 × 105 TCID50 of SARS-CoV-2. Live virus titres (e) and viral load of SARS CoV-2 (f) in nasal washes obtained from ferrets according to the indicated timeline after challenge. The dotted line indicates the highest value measured in the normal control group, which was 2.125 log10 N copy number/mL in the nasal washes. The statistical significance was assessed using a Mann–Whitney test; p values less than 0.01 were considered to be statistically significant where ** denotes 0.01; ns: not significant. Error bars represent mean ± standard deviation.