Figure 2.
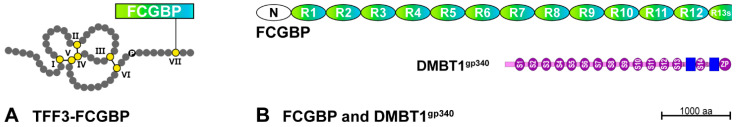
Schematic structures of the human TFF3-FCGBP heteromer (A) as well FCGBP and DMBT1gp340 (B). (A) TFF3 forms a three-leafed structure (TFF domain) by three disulfide bridges between CysI to CysVI. The 7th cysteine residue is linked to the high molecular mass glycoprotein FCGBP (see panel B) via a disulfide bridge (not drawn to scale). Cysteine residues are shown in yellow. Additionally represented is a characteristic proline residue (P) separating the TFF domain from CysVII. (B) Schematic structures of the high-molecular mass glycoproteins FCGBP and DMBT1gp340. Outlined are the various modules; some of them are repetitive and cysteine-rich, e.g., R1-R13s, S1-S14 (SRCR domains). Sale bar: 1000 amino acid residues.