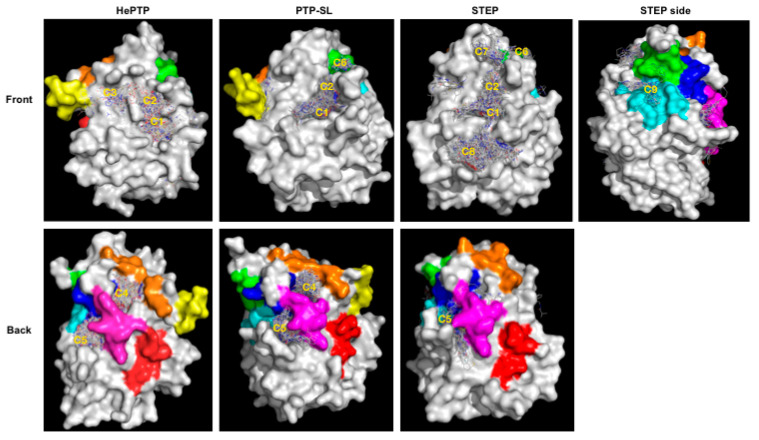
Figure 1.
Blind docking results for kinase interaction motif protein tyrosine phosphatases KIM-PTPs (HePTP, PTPSL and STEP). The structure of the three KIM-PTPs are shown as a grey surface with key functional KIM-PTP specific motifs [33] coloured as follows: K2 in yellow, K3 in orange, K4 in red, K5 in magenta, K6 in cyan, K7 in blue, K8 in green. Compounds are shown as lines. Areas where there is a high density of compounds (greater than 10) are classified as clusters and numbered: C1, active site; C2, open form pocket; C3, substrate-binding site; C4, centred between K3, K5 and K7; and C5, centred on K5, K6 and K7; C6, centred on K8; C7 above open pocket; and C8 below the active site. The front view and back view for all three KIM-PTPs are shown. For STEP, a side view is also shown to see cluster C9 on K6.