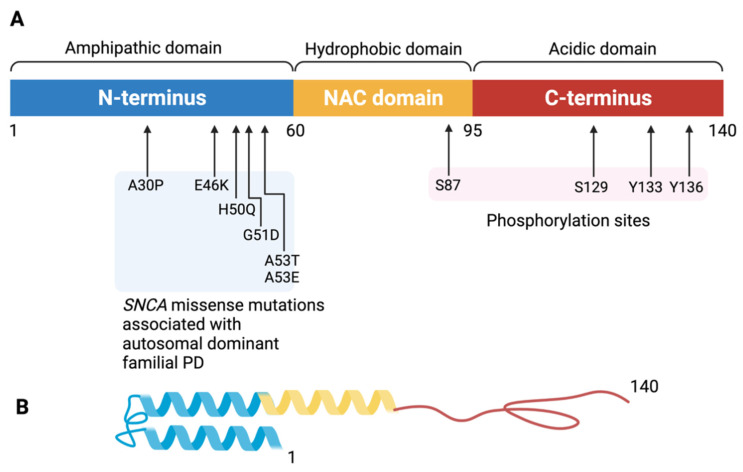
Figure 1.
The structure of the alpha-synuclein monomer. (A) Schematic depiction of alpha-synuclein structure. The amino acid residues delimiting the N-terminus, NAC region, and C-terminus as well as those that are sites of known mutations are labeled. The 140-amino-acid protein can be divided into three distinct domains. The N-terminal amphipathic domain (in blue) contains the amino acid residues affected by the main alpha-synuclein gene mutations (A30P, E46K, H50Q, G51D, A53T, A53E) associated with autosomal dominant Parkinson disease. The N-terminal region has a helical folding propensity and is responsible for membrane binding. The hydrophobic non-amyloid β-component of plaque (NAC) domain (in yellow) is responsible for promoting aggregation. The C-terminal domain (in red) forms an acidic tail containing the main phosphorylation site at Ser129. The C-terminal domain modulates alpha-synuclein aggregation. (B) Tertiary structure of the α-synuclein monomer. Created with BioRender.com (accessed on 12 October 2021).