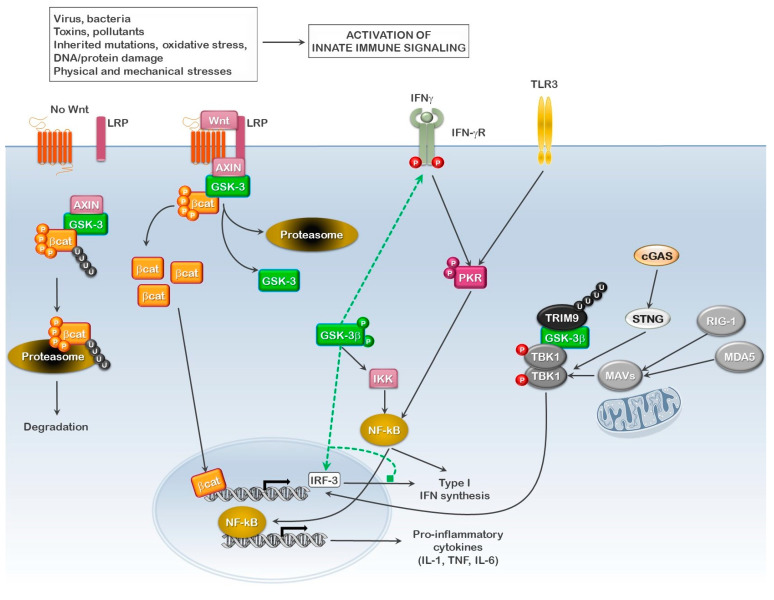
Figure 3.
Role of GSK3 in regulating innate immune signaling. Synthesis of type I interferons requires both interferon response factor (IRF), NF-κB activation and nuclear localization, and the subsequent binding to their respective elements in the IFNα/β promoter. In the presence of active GSK3, β-catenin is phosphorylated and degraded, thus inhibiting the synthesis of IRF-3, while NF-κB activation and nuclear localization are stimulated, thus favoring the synthesis of pro-inflammatory cytokines. In addition, active GSK3 also stabilizes the IFNγ receptor (IFNγR), thus enhancing IFNγ pro-inflammatory signaling. In the absence of GSK3 activity, β-catenin is released upon stimulation, translocates to the nucleus and promotes the synthesis of IRF-3. Signaling mediated by the pattern recognition receptor (PRR) proteins (RIG-I, MDA5, cGAS) promotes IRF-3 activation and nuclear localization, while the activation of PKR results in p65 NF-κB phosphorylation, activation and nuclear translocation where active p65 NF-κB either synergizes with signaling mediated by the other PRRs to aid in the synthesis of type I IFN (GSK3 inactive) or induces the synthesis of pro-inflammatory cytokines (GSK3 active).