Abstract
Background
Mass indoor gatherings were banned in early 2020 to prevent the spread of SARS-CoV-2. We aimed to assess, under controlled conditions, whether infection rates among attendees at a large, indoor gathering event would be similar to those in non-attendees, given implementation of a comprehensive prevention strategy including antigen-screening within 3 days, medical mask wearing, and optimised ventilation.
Methods
The non-inferiority, prospective, open-label, randomised, controlled SPRING trial was done on attendees at a live indoor concert held in the Accor Arena on May 29, 2021 in Paris, France. Participants, aged 18–45 years, recruited via a dedicated website, had no comorbidities, COVID-19 symptoms, or recent case contact, and had had a negative rapid antigen diagnostic test within 3 days before the concert. Participants were randomly allocated in a 2:1 ratio to the experimental group (attendees) or to the control group (non-attendees). The allocation sequence was computer-generated by means of permuted blocks of sizes three, six, or nine, with no stratification. The primary outcome measure was the number of patients who were SARS-CoV-2-positive by RT-PCR test on self-collected saliva 7 days post-gathering in the per-protocol population (non-inferiority margin <0·35%). This trial is registered with ClinicalTrials.gov, NCT04872075.
Findings
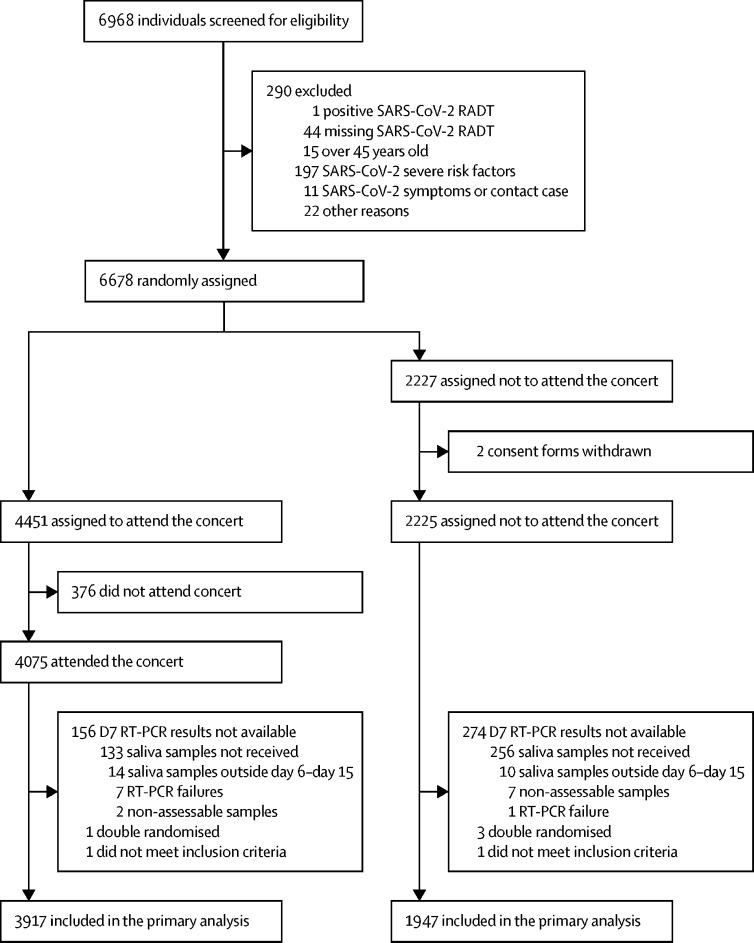
Between May 11 and 25, 2021, 18 845 individuals registered on the dedicated website, and 10 953 were randomly selected for a pre-enrolment on-site visit. Among 6968 who kept the appointment and were screened, 6678 participants were randomly assigned (4451 were assigned to be attendees and 2227 to be non-attendees; median age 28 years; 59% women); 88% (3917) of attendees and 87% (1947) of non-attendees complied with follow-up requirements. The day 7 RT-PCR was positive for eight of the 3917 attendees (observed incidence, 0·20%; 95% CI 0·09–0·40) and three of the 1947 non-attendees (0·15%; 0·03–0·45; absolute difference, 95% CI −0·26% to 0·28%), findings that met the non-inferiority criterion for the primary endpoint.
Interpretation
Participation in a large, indoor, live gathering without physical distancing was not associated with increased SARS-CoV-2–transmission risk, provided a comprehensive preventive intervention was implemented.
Funding
French Ministry of Health.
Translation
For the French translation of the abstract see Supplementary Materials section.
Introduction
The COVID-19 pandemic has halted all major gatherings in enclosed environments without physical distancing. As its incidence was surging worldwide, and evidence accumulating on the transmission risk from asymptomatic carriers1 and airborne droplets,2 most health-care authorities banned mass, indoor gatherings in early 2020. Mass-gatherings have led to further spread of SARS-CoV-2 at community and national levels.3, 4, 5, 6
Early so-called super-spreader events,6, 7, 8, 9, 10, 11 with no mask-wearing and activities favouring high aerosol production (eg, choirs, nightclubs, and religious meetings), were reported in venues poorly ventilated or with recirculated air. Because live indoor concerts with a standing audience meet all those conditions, they were identified as highly risky environments for spreading SARS-CoV-2. The French government decreed a general ban on gatherings of greater than 5000 people on Feb 28, 2020, which was subsequently extended to all gatherings on March 17, 2020. The events industry has come to a standstill, with major economic and psychological consequences, especially among the youngest. After 15 months of closure, and with highly efficient vaccines available, the question of reopening concert halls has emerged.
Several experiments in Germany, the Netherlands, the UK, and Spain, tried to define optimal interventions to prevent SARS-CoV-2 spread during events without physical distancing.12, 13, 14 Most were observational, except one randomised controlled trial during an indoor live concert held in Barcelona, Spain, in December, 2020;12 the concert included only half (n=465) the normal participants who underwent same-day rapid antigen diagnostic tests (RADTs) and wore N95 masks, which are impractical constraints for large gatherings.
Research in context.
Evidence before study
Since February, 2020, the COVID-19 pandemic has put a stop to most major gatherings without physical distancing in enclosed environments. Very few studies have attempted to define optimal conditions for holding such events again. In July, 2021, we searched PubMed, using the search terms “COVID-19”, “mass-gathering”, and “prevention”. The search found six studies, mainly observational. One single randomised controlled trial, done during an indoor live concert in Barcelona in December, 2020, provided preliminary evidence that same-day rapid antigen diagnostic testing and N95 mask wearing would prevent transmission. However, the number of participants was greatly restricted, and some logistic problems were encountered in implementing testing on the event day, making that approach difficult for larger events.
Added value of this study
The SPRING (Study on Prevention of SARS-CoV-2 Transmission at a Large Indoor Gathering) is, we believe, the first randomised, controlled trial testing the hypothesis that systematic SARS-CoV-2 screening within the 3 days preceding the event and simple medical mask wearing by participants can prevent SARS-CoV-2 transmission for concerts held in enclosed spaces, at full capacity, with a standing audience. Overall, 6678 participants were randomly assigned (4451 in the experimental group and 2227 in the control group). The trial showed that participating in such an event was not associated with an increased risk of SARS-CoV-2 transmission.
Implications of all the available evidence
The results of the SPRING trial shed light on the conditions required to resume live concerts and other indoor mass-gatherings. Until herd immunity is achieved and in a context of ongoing circulation of highly transmissible SARS-CoV-2 variants, resumption of cultural activities can be envisaged, provided a comprehensive preventive intervention, including testing within 3 days preceding the event and mask wearing, are implemented.
The hypothesis underlying the Study on Prevention of SARS-CoV-2 transmission in a large INdoor Gathering (SPRING) trial was that a strategy combining systematic antigen screening within 3 days preceding the event, medical mask wearing, and optimised ventilation could prevent SARS-CoV-2 spread during a large, indoor gathering without physical distancing.
Methods
Study design and participants
This prospective, open-label, non-inferiority randomised (2:1), controlled trial was done during a live indoor concert on May 29, 2021 held in the Accor Arena, Paris, France. All participants were recruited via official (Public Assistance—Paris Hospitals [APHP] and PRODISS) and non-official media (Twitter, Facebook, Linkedin, artists websites, and social networks), leading them to a dedicated website. Participants, randomly drawn from that website, were invited to a pre-enrolment visit during the 3 days preceding the event at the concert venue, where 116 clinical research assistants verified their eligibility. Adults aged 18–45 years, residing in the Paris region, with no relevant comorbidities, not living with older or at-risk people, and a negative SARS-CoV-2 RADT within the 3 days preceding the event, were eligible. The nasopharyngeal swabs SARS-CoV-2 RADT were done on participants by health-care professionals, with results transmitted within 15 min, by an automatically generated text message. SARS-CoV-2–positive participants were entered into the national contact-tracing programme. Participants reporting COVID-19–suggestive symptoms, or contact with a laboratory-confirmed infection within the 14 days preconcert were excluded. Detailed inclusion and non-inclusion criteria are provided in appendix 2 (p 2). The trial protocol was approved by the Scientific Ethics Committee of the Sud-Ouest and Outremer Regions of France and the French Data-Protection Agency and was registered on ClinicalTrials.gov (NCT04872075). All participants provided written informed consent.
Randomisation and masking
Test-negative participants were enrolled and randomly assigned in a 2:1 ratio to be in the experimental group (attendees) or in the control group (non-attendees). The allocation sequence was computer-generated by means of permuted blocks of sizes three, six, or nine, with no stratification. The allocation sequence was concealed from the research team assigning participants to groups and randomisation was achieved by means of a centralised, secure, interactive, web-response system (Cleanweb, Telemedecine Technologies SAS, Boulogne-Billancourt, France). Study participants were informed of their randomisation group by text message and email within 24 h of their inclusion. Participants allocated in the experimental group returned to normal life after the event. At the time of event, mask wearing was mandatory outdoors and indoors and a curfew was imposed at 2100 h. This was an open-label study in which both participants and investigators knew to which groups participants had been randomly assigned.
Procedures
Before entering the arena, attendees presented their negative RADT results. At the entrance gates, all received a medical face mask, mandatorily worn throughout the event, and hydroalcoholic hand-sanitisation was required, with sanitiser available at multiple sites inside. Water bottles were distributed, with mask removal allowed only for drinking. Bars were closed and alcoholic beverages were not permitted. In-arena movements were directed and controlled by the security team.
The arena's total area is 55 000 m2, for maximal capacity of 20 000 attendees (12 300 seated, 5700 standing on the floor, and 2000 elsewhere). During the event, only the floor was accessible to participants (1900 m2). Ventilation was supplied by eight air-handling units working with 100% outside air, and no recirculated air. Ventilation was located in the arena floor and in all the parts receiving the public (box seats, corridors, staircases, and restrooms) and was initiated at least 3 h before the entry of the public on site. The system is sized to ensure air renewal in situations where the concert hall is opened at full capacity (20 000 attendees). Considering that less than 5000 participants were present during the event, the ventilation can be considered as optimised. No physical distancing was required in the concert room (full capacity of the floor arena was 5700); singing and dancing were permitted.
Doors were open from 1600 to 2000 h for the 2·5 h show, included two performances: one DJ session and one live music. All artists and staff members (n=525) were tested for SARS-CoV-2 within the 3 days preceding the event and were not tested at follow-up.
All randomly assigned participants were given two self-saliva-collection kits. The first, collected on event day (day 0), was returned by attendees at the gate and mailed in prepaid envelopes by non-attendees; the second, collected on day 7 (ie, day 6–day 15 window) post-event, was mailed by all participants in prepaid envelopes. All samples were centralised and processed at the high-throughput platform of the Assistance Publique–Hôpitaux de Paris. All participants tested positive were contacted individually by a medical team member to collect clinical information and initiate contact tracing. Participants with clinical symptoms appearing between day 0 and day 15 were told to contact their primary physician, and notify the researchers of any additional screening-test results. Study participants were encouraged to comply with trial procedures with incentives (discounted ticket for attendees or a free ticket for a future concert for non-attendees). Participants received regular reminders (text messages and phone calls) until day 15 to send their samples.
Standard Q COVID-19 Ag test (SD Biosensor, Roche Diagnostics, Meylan, France) was run on nasopharyngeal samples for screening within 3 days before the event. RT-PCR on day 0 and day 7 saliva samples followed the procedure for TaqPath COVID-19 CE IVD RT PCR Kit (Thermo Fisher Scientific, France). We previously showed that sensitivity of RADT was 93% and saliva RT-PCR was 85% in a population of individuals attending COVID-19 community screening centres.15 Whole-genome-sequencing was done for SARS-CoV-2 subtyping and molecular analysis of transmission clusters (appendix 2 p 2).
On entering the arena, five cameras, located in the lobby, staircases, and floor, captured video recordings (appendix 2 p 3). Recordings were processed with the Datakalab computer algorithm (Paris, France), which uses convolutional neural networks to, first, detect faces on each frame of a given video, then to predict mask wearing or not, designated as: no mask—the face has no mask, or wears it under the chin; inadequate—the mask does not cover the nose; and adequate—the mask covers both the mouth and nose. When the algorithm was unable to classify a face, it was designated as unassigned.
Mask-wearing compliance is expressed as the number of attendees adequately wearing their masks divided by the total number of faces counted on video captures throughout the concert. Information collected by the different cameras was summed by crosslinking the data corresponding video-recording times. The percentage of adequately masked faces was calculated per 5-min increments. The overall statistic was calculated over the total 4-h period. Subanalyses were computed by considering only circulation-area or arena-floor cameras.
Outcomes
The primary outcome was the number of SARS-CoV-2-positive RT-PCR tests on self-collected saliva at day 7 (ie, day 6–day 15 window) post-gathering according to the viral RNA kinetic16, 17, 18 and to the protocol of previous similar studies.12 This was analysed for the per-protocol population (complete case analysis), which included all randomised, eligible participants without any major protocol deviations. Major protocol deviations were missing day 7-saliva RT-PCR result, day 7-saliva swab obtained outside the day 6–day 15 window, double randomisation and attendees who did not come. The main analysis yielded the absolute positivity-rate differences 95% CI between the two groups.
The secondary outcomes were the conversion rate of salivary carriage between the day 0 and day 7 visits and the percentages of adequately masked (nose and mouth covered) faces over the total 4 h period gathering and according to location in the venue calculated by means of an artificial intelligence tool analysing anonymised, continuous video-capture data.
Statistical analysis
We hypothesised that concert attendance in a closed arena would not engender increased risk of SARS-CoV-2 contamination compared with non-attendees, whose expected COVID-19-incidence rate 1 week later would not exceed 200 per 100 000 inhabitants (appendix 2 p 4). To satisfy the non-inferiority hypothesis, the upper limit of the two-sided 95% CI of the absolute difference of contamination rates (ie, attendees minus non-attendees) had to be less than 0·35%. That non-inferiority margin was chosen as the maximum clinically acceptable difference. According to a 2:1 ratio and assuming equal contamination rates in both groups, 4500 attendees and 2250 non-attendees provided 85% power. By anticipating 10% loss-to-follow-up rate for the primary endpoint, it was planned to randomly assign 5000 attendees and 2500 non-attendees. Non-inferiority intention-to-treat (ITT) and sensitivity analyses assessed result robustness (appendix 2 p 4). The Miettinen and Nurminen method implemented in the gsDesign package was used to construct CIs of the difference in contamination rates as well as for those of the incidence rate ratios (IRRs).19
Categorical data are expressed as numbers (percentages) and continuous data as median (IQR). Statistical analyses were computed with R-4.0.3 software and the gsDesign package. This trial is registered with ClinicalTrials.gov, NCT04872075.
Role of the funding source
The funders had no role in the study design or conduct of the trial, and had no role in data collection, data analysis, writing of the report, or the decision to submit for publication.
Results
Between May 11 and 25, 2021, 18 845 individuals registered on the dedicated website, and 10 953 were randomly selected for a pre-enrolment on-site visit. Among 6968 who kept that appointment and were screened for inclusion (May 26–28, 2021; figure 1 ), 290 (4·2%) did not fulfil the inclusion criteria; only one of them was RADT-positive. Among 6678 randomly assigned participants, 4451 were assigned to be attendees and 2227 to be non-attendees; 376 (8·4%) did not attend and 158 (3·5 %) had major protocol deviations, with the result that the primary analysis concerned 3917 attendees. Major protocol deviations of 280 (12·6%) non-attendees left 1947 for primary outcome assessment.
Figure 1.
Trial profile
RADT=rapid antigen diagnostic test.
The median age of primary analysis participants was 28 years (IQR 24–35); 59·0% were females (table ). 43·1% of participants were vaccinated with one dose and 7·2% with two doses. Median (IQR) of days between first dose and inclusion was 14 days (7–26) and between second dose and inclusion was 26 days (16–46). Among the 5864 participants, 335 (5·7%) had received two doses at least 14 days previously and were considered fully protected.
Table.
Baseline characteristics
| Attendees (n=3917) | Non-attendees (n=1947) | ||
|---|---|---|---|
| Age | 28 (24–35) | 28 (24–35) | |
| Sex | |||
| Female, | 2312 (59·0%) | 1137 (58·4) | |
| Male | 1605 (41·0%) | 810 (41·6%) | |
| Declared COVID-19 history | 681 (17·4%) | 343 (17·6%) | |
| Vaccinated | |||
| No | 1943 (49·6%) | 970 (49·8%) | |
| One dose | 1684 (43·0%) | 844 (43·3%) | |
| Two doses | 290 (7·4%) | 133 (6·8%) | |
| Inclusion-to-first-dose interval, days | 14 (8–26) | 14 (7–25) | |
| Inclusion-to-second-dose interval, days | 26 (15–42) | 27 (17–56) | |
Data are n (%) or median IQR.
Baseline characteristics of the randomised intention-to-treat population are provided in appendix 2 (p 6).
Day 7 RT-PCRs were positive for eight of the 3917 attendees (observed incidence 0·20%; 95% CI 0·09 to 0·40) and three of the 1947 non-attendees (0·15%; 95% CI 0·03 to 0·45). The 95% CI of the positivity-rate absolute difference between attendees and non-attendees groups was −0·26% to 0·28%. The upper limit of that CI did not exceed the prespecified, non-inferiority margin (0·35%), thereby confirming non-inferiority. The IRR was estimated to be 1·33 (95% CI 0·38 to 4·60).
Among 14 participants with positive saliva at day 0 and available sample at day 7, eight (57%; 95% CI 29 to 82) were negative on day 7, suggesting that they were at a late stage of infection (and not detected by the RADT; appendix 2 p 10). Five of eight attendees with day 7-positive RT-PCRs had already been RT-PCR–positive on day 0, thereby excluding their contamination during the concert. But cycle threshold values decreasing between day 0 and day 7 suggest that they were at the onset of infection with a potential risk of transmission to others attendees during the concert. Clinical characteristics and day 0 test and day 7 test results of RT-PCR-positive participants are reported in appendix 2 (p 7).
Post-event, six participants reported mild COVID-19-suggestive symptoms (four attendees and two non-attendees). None with RT-PCR-positive saliva required hospitalisation.
Non-inferiority ITT analysis of five imputed datasets and sensitivity analyses confirmed the primary analysis conclusion: all 95% CI upper limits were below the 0·35% non-inferiority margin (appendix 2 p 9). Viral subtyping and the transmission analysis cluster are presented in appendix 2 p 10.
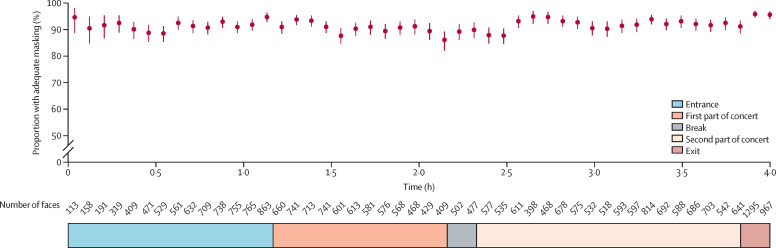
Throughout the event, the computer algorithm detected 33 349 faces; 28 302 (85%) were classified as no mask, inadequate, or adequate. Overall median mask-wearing compliance was estimated at 91·4% (95% CI 87·7 to 95·4): 90·0% (76·5 to 94·8) on the arena floor and 97·4% (74·1 to 99·9) in the lobby and staircases, and remained stable over the 4 h period (figure 2 ).
Figure 2.
Overall mask-wearing compliance during the gathering
Red circles represent the percentages of adequately masked (nose and mouth covered) faces, calculated for increments of 5 min. Red vertical lines represent the 95% CI for each value.
Discussion
In an indoor arena in which the audience tested negative, showed high compliance with medical mask wearing, and ventilation was optimised, transmission of SARS-CoV-2 was rare. Eight of the 3917 attendees became infected, compared with three of the 1947 non-attendees, thereby confirming the absence of transmission risk. Overall mask-wearing compliance, estimated with a computer algorithm, was 91·4% throughout the 4 h event.
To our knowledge, no randomised controlled trial enrolling so many participants with sufficient statistical power to estimate transmission risk at a live indoor concert has been reported. Several published, mostly observational, studies have attempted to define optimal conditions for reauthorising such gatherings: upstream SARS-CoV-2 testing, mandatory facemasks, optimal ventilation, encouraging hand-sanitiser use, surface cleaning, and flow management during the event. An experimental event in Leipzig, Germany, in August, 2020, including 1212 participants and applying three hygiene concepts (all testing negative, wearing N95 masks, and different seating patterns) showed that seated indoor events, with implemented precautions and adequate ventilation, had little effect on SARS-CoV-2 spread.14
To date, only one much smaller (n=465) randomised controlled trial of an indoor live concert in Barcelona, Spain, in December, 2020, evaluated the efficacy of a comprehensive preventive intervention on the basis of a systematic same-day RADT-screening of attendees, compulsory N95 facemask wearing, and adequate site ventilation.12 That trial provided preliminary evidence of point-of-care RADT-screening, mask wearing, and air-ventilation efficacy at preventing SARS-CoV-2 transmission during an indoor mass-gathering event. Those authors' observational study, on 5000 attendees under the same hygiene procedures on March 27, 2021, had an observed 0·13% incidence rate among attendees (six positive among 4584 within the 2 weeks post-gathering) similar to the background population based on public health records.13 That result confirmed their randomised controlled trial outcome on a real-life number of attendees but the observational design precluded drawing definitive conclusions.
However, RADT implementation for all attendees on the concert day proved to be a major logistic problem, making it unenforceable for larger events. Furthermore, N95 masks are usually restricted to professional use, and might be uncomfortable and inappropriately worn by the general public, particularly over extended periods and during concerts.
The strengths of our RCT were inclusion of quasi-full capacity (n=4451) participants at a single event; RADT-screening within the 3 days preceding the event, rather than day 0; required medical mask wearing (not N95); and the very challenging obtention of day 7-saliva RT-PCR samples from both groups for primary outcome success, with very high day 7-test-adhesion rates owing to incentives, text messages, phone reminders, gifts, and easy-to-collect and mail saliva samples. Retrieval of saliva samples was actually a major challenge to ensure success of the experiment. All participants were reminded to return their saliva samples by automatic text messages and emails on day 3, day 6, day 8, and day 10. Additionally, the clinical research team individually contacted those who had not sent their day 0 and day 7 samples by phone on day 8–day 9 (n=3026 including the 704 participants who were lost to follow-up).
Notably, day 0-saliva RT-PCRs to estimate the number of participants already positive showed that five of the eight preconcert, RADT-negative attendees were also positive on day 7. The false-negative RADT-screening could reflect very early infection or a lower RADT sensitivity. RADT-screening on pre gathering day −1, day −2, or day −3 had no effect on the risk of false-negative results but the case numbers were low. RADTs are less sensitive than RT-PCR on nasopharyngeal and saliva samples,15, 20, 21 being only able to estimate infectious people, except for 1 or 3 days post-infection, whereas RT-PCRs remain positive for several weeks after the infectious period.22 Pertinently, whole SARS-CoV-2 genome-sequencing examined whether day 7 RT-PCR–positive attendees were contaminated with the same strain.23 The absence of transmission clusters, based on phylogenetic analyses, was confirmed for the five attendees with available sequences, but this result was limited by a low number of cases. Contact tracing did not show other contamination risk (such as public transport) in the positive participants in either group, but at the end of May, 2021, the curfew shifted from 1900 h to 2100 h, restaurants opened outdoors with no more than six people, gatherings with more ten people were banned, and mask wearing was mandatory outdoors and indoors. Epidemiological and contact tracing data with phylogenetic analysis or, more recently, mobility data demonstrated the role of mass gathering events as predominant initial sources of cases. 11, 24
The non-inferiority analyses confirmed the primary analysis finding and reinforced the absence of enhanced virus-transmission risk. Moreover, primary analysis incidence rates for the two participant groups were similar (0·20% for attendees and 0·15% for non-attendees), and were consistent with the concomitant, age-standardised, 14-day, cumulative incidence rate of 190 cases per 100 000 Paris-region inhabitants.
Mask wearing is now recognised as a major measure preventing SARS-CoV-2 transmission, including in community settings.25 However, adequate mask-wearing compliance (ie, covering nose and mouth) by the general public over a prolonged period has rarely been assessed.26 In health-care settings, compliance estimation typically relies on direct observation of health-care personnel by trained investigators (practice audits), whereas community studies analysed airport video-surveillance-footage photographs.27, 28 However, such evaluations of large populations encounter logistical difficulties because manual counting by observers is unfeasible. Artificial intelligence technology was used in the Paris subway to study people's mask-wearing compliance on continuous captured-video data, but the special lighting effects of a live concert had not been attempted previously. Our results showed that overall mask-wearing compliance was high (estimated at 91·4%) and stable over the 4 h gathering. Interestingly, compliance was 90·0% on the floor (probably reflecting some breaks in order to drink), and 97·4% in the lobby and staircases during arena entry and exit. Medical masks efficiently prevented SARS-CoV-2 diffusion on the arena floor from the five saliva-positive attendees.
Our study has some limitations. First, participants' behaviour might have been modified because camera observation during this test concert was known. This phenomenon, intrinsic to clinical trials, can limit result extrapolation to a real-life scenario. Nevertheless, outdoor and indoor mask wearing was mandatory during the event and attendees were quite compliant awaiting entry. Notably, half the attendees had received at least one vaccine dose during the preceding 2 weeks, whereas the country-level anti-COVID-19 vaccination rate (one dose) was around 30% in the same age-range. That observation suggests attendees' awareness of hygiene protocols necessary to recover normal life activities. The rate of fully vaccinated people was 7·2% and similar between groups. The greater the proportion of immunisation, the lower the incidence of SARS-CoV-2 transmission during the gathering will be. Second, the infection incidence in France was decreasing in late May, 2021 and most infections were due to the SARS-CoV-2 alpha variant, less transmissible than the delta variant, which is circulating worldwide. Nevertheless, antigen testing accurately detects the delta variant and mask wearing seems efficient to prevent transmission, suggesting that the two main preventive measures would remain effective. However, a study published in 2021 reported that the delta variant had a shorter incubation period and a higher viral load compared with the original strain, suggesting that people might start spreading the virus earlier after they become infected.29 Antigen testing within 3 days preceding the gathering might not be enough to prevent the transmission during the concert. Further studies on preventive measures during large gatherings are needed, which account for the highly transmissible emerging variants, to confirm our results. Third, the statistical analyses done in this study also have limitations, in particular on the choice of analysis populations. Although, the primary and sensitivity analyses explore many scenarios in the dataset, these analyses excluded participants on the basis of post-randomisation information (ie, major protocol deviations or missing data for the primary outcome), which cannot guarantee the presence of collider bias.30 For example, the exclusion of participants who have not returned their saliva samples might have led to an erroneous estimation of the SARS-CoV-2 positivity rate in either direction. To overcome the methodological limitations of per-protocol analysis sets and in accordance with the statistical analysis plan, we also provide the results obtained in the ITT population. However, contrary to superiority trials, ITT analyses in non-inferiority trials could be less conservative and tend to bias the results towards the alternative hypothesis. In addition, the imputation method used might also influence the conclusions. Per-protocol and ITT analyses could have methodological bias; however, all the analyses done in this study concluded non-inferiority, which suggest the robustness of our results (supplemental references, appendix 2 p 5). Fourth, cluster randomisation might have improved the attendance rate, since 8·5% of the designated group did not attend. Finally our study has been done for healthy young people whereas superspreading outcomes could be different for another population.31, 32
In conclusion, our results shed light on the conditions required to resume live concerts and other indoor mass-gatherings. Until herd immunity is achieved, our findings showed that participation in a large, indoor, live concert without physical distancing was not associated with heightened SARS-CoV-2-infection risk, provided comprehensive preventive measures were implemented. In the context of low circulation of SARS-CoV-2, it is now possible to envisage resumption of cultural activities applying health-protocol adaptations. In the case of highly transmissible variants with shorter incubation period, mask wearing should be maintained. Immunisation coverage, not studied here, might also affect the control of the viral circulation during large gatherings.
For Paris COVID-19 data see https://www.santepubliquefrance.fr
Data sharing
The complete de-identified participant dataset will be available on request to fabric.gourmelon@aphp.fr for researches whose proposed use of the data has been approved, for any purpose. Data will be available with publication. Additional documents (study protocol, statistical analysis plan, informed consent form) will also available if needed with publication.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
Acknowledgments
The trial was supported by a grant from the French Ministry of Health for the scientific part. The Region Ile de France and private and professional partners supported the production part. We thank the participants for their contribution to the SPRING study. We thank the members of the Department of Clinical Research and Innovation, Direction des Sytèmes d'Information, service de presse and COVISAN of Assistance Publique-Hôpitaux de Paris for their role in the recruitment and the import of the dataset; the staff of the plateforme COVID-IDF and SALICOV team research for performing PCR tests on saliva samples; the staff of URC Cochin and Cleanweb for the inclusion visits and dataset management; the national mail La poste and IMEA for the saliva sample mailing; the Roche Diagnostic Laboratories for providing antigenic tests free of charge; the Mission of European and International Affairs–Organisation of the Directorate-General for Health for providing the new version TousANticovid Wallet application; DATAKALAB staff for the analysis of mask-wearing compliance. We also thank the members of PRODISS, Weezevent, We love Green, Live Nation, Alias, and AccorArena and the artists for the organisation of the event. Finally, we thank the Marie de Paris, the Paris region, the Ministry of Health, the Ministry of Culture, and the ANRS-MIE agency for their support and Janet Jacobson for providing medical writing support.
Contributors
CD, SK, MSe, SS, JLG, XL, and JM-T conceptualised the study. SS, ST, and AO recruited participants and organised the venue framework. NG, SMD, MLN, MM, AG, and CP did the laboratory analyses on clinical samples. FF, HA, and JM-T did the statistical analyses. SMD and MSa did the whole genome sequencing and phylogenetic analyses. GM, LC, and ED, accessed and verified the data. CD, SK, FF, and HA wrote the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
SPRING study group
F Gourmelon (Unité de Recherche Clinique—Centre Investigation Clinique, AP-HP, Hôpital Necker-Enfants malades, Paris, France). C Leroy (Plateforme COVID IDF, AP-HP. Centre Université de Paris, Paris). X Fischer (Datakalab, Paris, France). C Taille (Direction des Systèmes d'Information, AP-HP, Paris, France).
Supplementary Materials
References
- 1.Wilmes P, Zimmer J, Schulz J, et al. SARS-CoV-2 transmission risk from asymptomatic carriers: results from a mass screening programme in Luxembourg. Lancet Reg Health Eur. 2021;4 doi: 10.1016/j.lanepe.2021.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci USA. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Che Mat NF, Edinur HA, Abdul Razab MKA, Safuan S. A single mass gathering resulted in massive transmission of COVID-19 infections in Malaysia with further international spread. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandl M, Selb R, Seidl-Pillmeier S, Marosevic D, Buchholz U, Rehmet S. Mass gathering events and undetected transmission of SARS-CoV-2 in vulnerable populations leading to an outbreak with high case fatality ratio in the district of Tirschenreuth, Germany. Epidemiol Infect. 2020;148:e252. doi: 10.1017/S0950268820002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miron O, Yu K-H, Wilf-Miron R, Davidovitch N. COVID-19 infections following outdoor mass gatherings in low incidence areas: retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.10.22.20184630. published online Oct 27. (preprint) [DOI] [Google Scholar]
- 6.Gerbaud L, Guiguet-Auclair C, Breysse F, et al. Hospital and population-based evidence for COVID-19 early circulation in the east of France. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17197175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2021;31:314–323. doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahl P, de Silva C, Bhattacharjee S, et al. Droplets and aerosols generated by singing and the risk of coronavirus disease 2019 for choirs. Clin Infect Dis. 2021;72:e639–e641. doi: 10.1093/cid/ciaa1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Li C, Dong H, et al. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA Intern Med. 2020;180:1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenther T, Czech-Sioli M, Indenbirken D, et al. Investigation of a superspreading event preceding the largest meat processing plant-related SARS-Coronavirus 2 outbreak in Germany. SSRN. 2020 https://papers.ssrn.com/abstract=3654517 published online July 23. (preprint) [Google Scholar]
- 11.Chang S, Pierson E, Koh PW, et al. Mobility network models of COVID-19 explain inequities and inform reopening. Nature. 2021;589:82–87. doi: 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- 12.Revollo B, Blanco I, Soler P, et al. Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial. Lancet Infect Dis. 2021;21:1365–1372. doi: 10.1016/S1473-3099(21)00268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llibre JM, Videla S, Clotet B, Revollo B. Screening for SARS-CoV-2 antigen before a live indoor music concert: an observational study. Ann Intern Med. 2021 doi: 10.7326/M21-2278. published online July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The risk of indoor sports and culture events for the transmission of COVID-19. (Restart-19) medRxiv. 2020 doi: 10.1038/s41467-021-25317-9. https:/10.1101/2020.10.28.20221580v1 published online Oct 30. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeGoff J, Kernéis S, Elie C, et al. Evaluation of saliva molecular point of care for detection of SARS-CoV-2 in ambulatory care. medRxiv. 2021 doi: 10.1101/2021.06.12.21258811. published online June 22. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 17.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 20.Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21:e290–e295. doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity—a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 23.Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20:1263–1271. doi: 10.1016/S1473-3099(20)30562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stange M, Mari A, Roloff T, et al. SARS-CoV-2 outbreak in a tri-national urban area is dominated by a B.1 lineage variant linked to a mass gathering event. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks JT, Butler JC. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA. 2021;325:998–999. doi: 10.1001/jama.2021.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganczak M, Pasek O, Duda-Duma Ł, Świstara D, Korzeń M. Use of masks in public places in Poland during SARS-Cov-2 epidemic: a covert observational study. BMC Public Health. 2021;21:393. doi: 10.1186/s12889-021-10418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elachola H, Assiri AM, Memish ZA. Mass gathering-related mask use during 2009 pandemic influenza A (H1N1) and Middle East respiratory syndrome coronavirus. Int J Infect Dis. 2014;20:77–78. doi: 10.1016/j.ijid.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elachola H, Ebrahim SH, Gozzer E. COVID-19: Facemask use prevalence in international airports in Asia, Europe and the Americas, March 2020. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Deng A, Li K, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. medRxiv. 2021 doi: 10.1101/2021.07.07.21260122. published online July 23. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernán MA, Robins JM. Causal Inference: What if. Miguel Hernán's faculty website. 2012. https://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/ published online Oct 19.
- 31.Edwards DA, Ausiello D, Salzman J, et al. Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2021830118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau MSY, Grenfell B, Thomas M, Bryan M, Nelson K, Lopman B. Characterizing superspreading events and age-specific infectiousness of SARS-CoV-2 transmission in Georgia, USA. Proc Natl Acad Sci USA. 2020;117:22430–22435. doi: 10.1073/pnas.2011802117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete de-identified participant dataset will be available on request to fabric.gourmelon@aphp.fr for researches whose proposed use of the data has been approved, for any purpose. Data will be available with publication. Additional documents (study protocol, statistical analysis plan, informed consent form) will also available if needed with publication.