Figure 4. Rapamycin prevents lesion formation due to CCM LOF and KLF4 GOF in neonatal and adult mice.
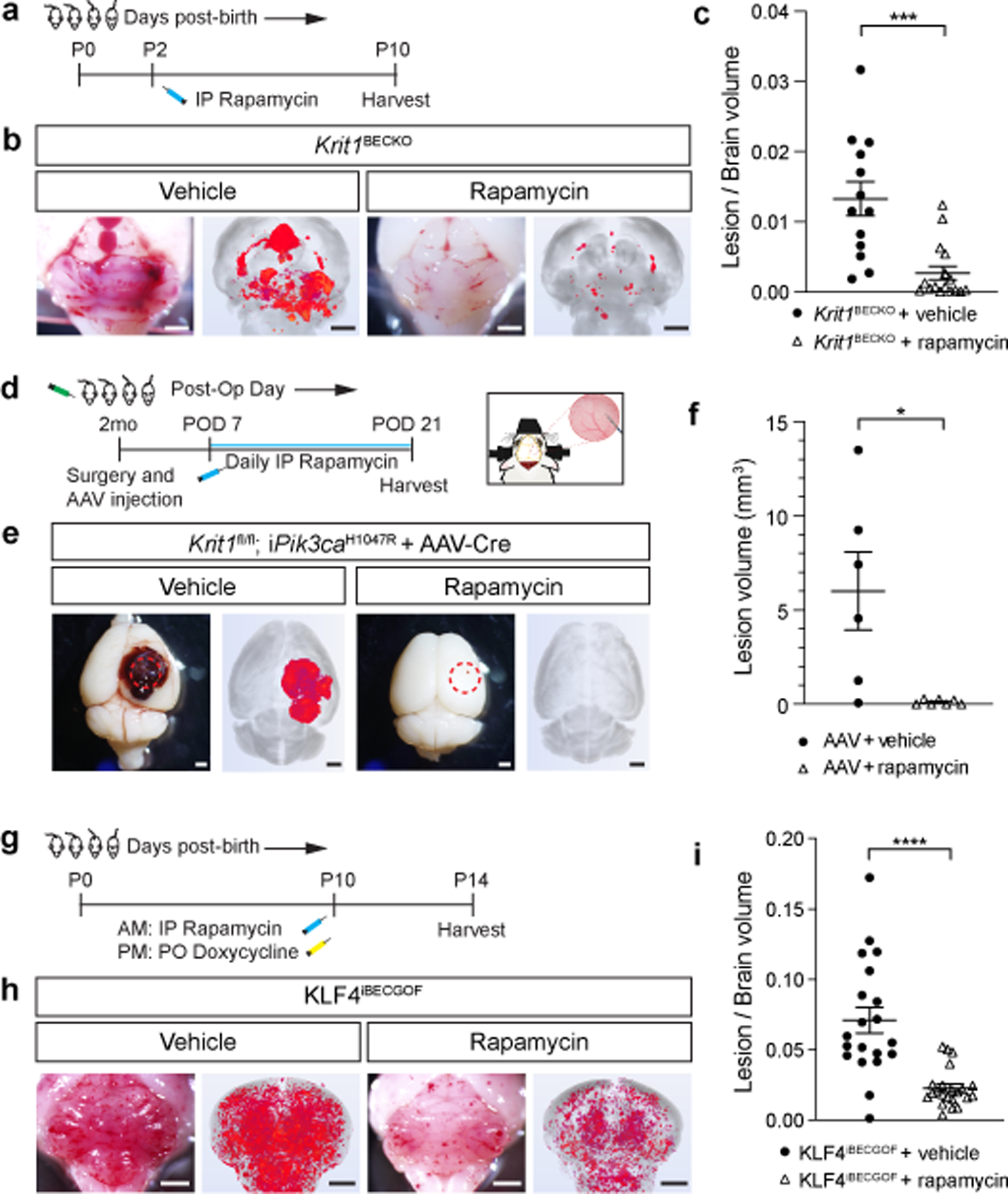
a, Schematic of neonatal Krit1BECKO animals with a susceptible microbiome administered a single dose treatment with Rapamycin or vehicle on P2. b, Representative visual and microCT images of the hindbrains of littermates treated with either vehicle or Rapamycin. Scale bars, 1mm. c, MicroCT quantitation of lesion volumes normalized to total brain volume following treatment with vehicle or Rapamycin. (Vehicle, n=13; Rapamycin, n=16). p=0.0009. d, Experimental design for Rapamycin or vehicle treatment of adult animals with combined CCM LOF and PIK3CA GOF (Krit1fl/fl;iPik3caH1047R) using cranial window surgery and AAV injection is shown. e, Representative visual and microCT images of brains harvested 21 days after injection of AAV-Cre into littermate animals. Dotted circles indicate the site of cranial window and AAV-Cre injection. Scale bars, 1mm. f, MicroCT quantitation of lesion volumes 21 days after creation of the cranial window and injection of AAV-Cre is shown. (Vehicle, n=6; Rapamycin, n=7). p=0.0358. g, Schematic of neonatal KLF4iBECGOF animals administered a single dose treatment with Rapamycin or vehicle followed by induction of KLF4 expression on P10. h, Representative visual and microCT images of the hindbrains of littermates treated with either vehicle or Rapamycin. Scale bars, 1mm. i, MicroCT quantitation of lesion volumes normalized to total brain volume following treatment of the indicated mice with vehicle or Rapamycin. (Vehicle, n=20; Rapamycin, n=22). p=4e−5. Data are mean ± s.e.m. Unpaired, two-tailed Welch’s t-test. *indicates p<0.05; ***indicates p<0.001; ****indicates p<0.0001.
