Abstract
Objectives
Patients with COVID-19 frequently develop acute respiratory distress syndrome (ARDS) requiring intensive care unit (ICU) admission. Data on long-term survival of these patients are lacking. The authors investigated 1-year survival, quality of life, and functional recovery of patients with COVID-19 ARDS requiring invasive mechanical ventilation.
Design
Prospective observational study.
Setting
Tertiary-care university hospital.
Participants
All patients with COVID-19 ARDS receiving invasive mechanical ventilation and discharged alive from hospital.
Interventions
Patients were contacted by phone after 1 year. Functional, cognitive, and psychological outcomes were explored through a questionnaire and assessed using validated scales. Patients were offered the possibility to undergo a follow-up chest computed tomography (CT) scan.
Measurements and Main Results
The study included all adult (age ≥18 years) patients with COVID-19–related ARDS admitted to an ICU of the authors’ institution between February 25, 2020, and April 27, 2020, who received at least 1 day of invasive mechanical ventilation (IMV). Of 116 patients who received IMV, 61 (52.6%) survived to hospital discharge. These survivors were assessed 1 year after discharge and 56 completed a battery of tests of cognition, activities of daily living, and interaction with family members. They had overall good functional recovery, with >80% reporting good recovery and no difficulties in usual activities. A total of 52 (93%) of patients had no dyspnea at rest. Severe anxiety/depression was reported by 5 (8.9%) patients. Comparing 2-month and 1-year data, the authors observed the most significant improvements in the areas of working status and exertional dyspnea. One-year chest CT scans were available for 36 patients; fibrotic-like changes were present in 4 patients.
Conclusions
All patients who survived the acute phase of COVID-19 and were discharged from the hospital were alive at the 1-year follow up, and the vast majority of them had good overall recovery and quality of life.
Key Words: coronavirus disease 2019, post-intensive care syndrome, quality of life, acute respiratory distress syndrome, intensive care unit, pulmonary fibrosis, lung recovery
IN THE FIRST MONTHS OF 2020, Coronavirus Disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread all over the world, becoming pandemic and causing a surge of intensive care unit (ICU) admissions due to severe respiratory failure and acute respiratory distress syndrome (ARDS).1, 2, 3, 4
Previous studies performed on patients with non–COVID-19 ARDS showed that ARDS frequently is associated with long-term functional, psychologic, and cognitive impairment and reduced quality of life in ICU survivors.5, 6, 7, 8 A similar pattern has been described for other respiratory coronaviruses infections (SARS-CoV-1 and Middle East Respiratory Syndrome), as well as for the general ICU population.9, 10, 11, 12, 13, 14
Short-term mortality rate for COVID-19–related ARDS requiring invasive mechanical ventilation is around 50%.3 , 15 However, data on long-term mortality and quality of life in this setting are scarce.16 , 17 In addition, previous studies showed conflicting data on midterm recovery, with some studies suggesting an overall reduced quality of life and poor functional recovery,18 , 19 while others reported an overall good quality of life and better functional recovery compared with non-COVID-19–related ARDS.20
The authors’ group recently published the 2-month follow-up data of the cohort of invasively ventilated COVID-19 ICU survivors.20 Accordingly, the authors now have performed a 1-year follow-up study of the same cohort to investigate long-term mortality, quality of life, functional and psychological recovery of the patients, as well as computed tomography (CT) characteristics of the lungs.
Materials and Methods
Study Design and Setting
This study was part of the COVID-BioB study, a prospective observational study performed at IRCCS San Raffaele Scientific Institute, a 1,350-bed teaching hospital in Italy. The study was approved by the hospital Ethics Committee (protocol No. 34/int/2020) and was registered at ClinicalTrials.gov (NCT04318366).21 , 22
Inclusion and Exclusion Criteria
Details on inclusion and exclusion criteria of the authors’ follow-up studies previously have been published.20 , 21
Briefly, this study included all adult (age ≥18 years) patients with COVID-19–related ARDS admitted to an ICU of the authors’ institution between February 25, 2020 and April 27, 2020 (first Italian pandemic wave23—see Supplementary Fig 1), who received at least 1 day of invasive mechanical ventilation (IMV). Of 116 patients who received IMV during the study period, 61 (52.6%) survived to hospital discharge. These survivors were contacted 1 year after discharge and were given a battery of tests of cognition, activities of daily living, and interaction with family members (details on data collection are provided below).
Patient Management
Details on hospital organization and patient management in the ICU and general wards previously have been published.4 , 20, 21, 22 , 24, 25, 26 Invasive mechanical ventilation, fluid management, and pharmacologic management of patients were performed according to international guidelines and expert recommendations.27, 28, 29, 30, 31
Briefly, the authors aimed for a tidal volume of 6-to-8 mL/kg of ideal body weight, a driving pressure of ≤15 cmH2O, and a pH > 7.25. Positive end-expiratory pressure initially was set according to the ARDSnet low positive end-expiratory pressure /high FIO2 table, and then individualized according to respiratory system mechanics, hemodynamics, and oxygenation. During the first days of ICU stay, the authors aimed for a slightly negative fluid balance as tolerated by hemodynamics and end-organ function. Rescue treatments, including prone positioning, use of neuromuscular blockade, inhaled nitric oxide, and extracorporeal membrane oxygenation, were used as suggested by international guidelines on ARDS management available at that time.27 , 31 Antivirals, adjuvants, and immunosuppressants (eg, steroids) were administered according to clinical judgment and evidence available at that time.21
Data Collection
Study methodology previously has been described.20 , 21 For this specific 1-year follow-up study, the primary outcome was 1-year survival. The authors chose survival as primary outcome, as this is a strong, clinically relevant outcome and there are no data on 1-year survival of patients with COVID-19–related ARDS who required IMV. All other items addressed by the follow-up questionnaire were secondary outcomes.
Baseline demographic and ICU treatment data were collected. For each patient, the authors had at least 1 telephone number, either belonging to the patient or to a caregiver. The discharged patients were contacted by phone by a trained investigator, and data were recorded progressively in a dedicated database during the phone interview. Firstly, the authors asked the patient to confirm his/her identity and date of birth, and then the authors asked further contact details and information about smoking habit at the onset of the disease (current smoker, past smoker >1 month, never smoker); working status at the onset of the disease (actively working or not); working status at the time of the call (back to previous job, working with different tasks due to the disease, not working for disease-dependent reasons, previously unemployed or retired); schooling (none, primary school, middle school, high school, bachelor's degree, master's degree); dysosmia and dysgeusia, either before the onset of the disease and after discharge; and discrimination that they (or their families) may have endured because of the disease, including denied access to nonurgent care. After that, the authors conducted a telephone assessment composed of various items, to explore multidimensional outcomes. The questionnaire was the same used for the 2-month follow-up,20 and items are described in details below and in the Supplementary Appendix. Finally, a chest CT scan was offered to all patients. Data were recorded progressively in a dedicated database during the phone interview.
Quality-of-Life Evaluation
Multidimensional outcomes were evaluated following the previous protocol.20 The authors explored physical recovery and disability via the Glasgow Outcome Scale extended (GOSe),32 , 33 autonomy in walking via the Functional Ambulation Classification,34 dyspnea (either at rest and during an effort such as 2 floors of stairs) via the Borg Category Ratio 10 scale,35 , 36 and nutritional status via the Mini Nutritional Assessment – Short Form (MNA-SF).37 The EuroQol 5 dimensions 3 levels (EQ-5D-3L),38 , 39 which includes an overall score self-evaluated by the patient, and the visual analog scale was used to assess quality of life. The authors used the Hospital Anxiety and Depression Scale,40 , 41 the Post-Traumatic Stress Disorder Checklist for DSM-5, which assesses posttraumatic stress disorder (PTSD),42, 43, 44 and the Insomnia Severity Index45 to assess psychological outcomes. The authors used the Italian Telephonic version of the Mini Mental State Examination to assess cognitive status.46
Chest CT Scans
All CT examinations were performed on a 64-row multidetector CT scanner (SOMATOM Definition Flash Dual Source CT, Siemens Healthcare). Computed tomography protocol included unenhanced, breath-hold axial scans of the thorax (from lung apex to the lowest hemidiaphragm); if any concern existed about air trapping,47 an expiratory scan also was performed.
Two experienced radiologists, both blinded to patients’ eventual symptoms, independently reviewed all CT images for fibrotic-like changes (honeycombing, reticulation, and traction bronchiectasis)47; differences in assessment were resolved with consensus.
Imaging postprocessing was carried out with commercially available software (Intellispace version 8.0, Philips Medical Systems, Chronic Obstructive Pulmonary Disease tool); after automated identification of pulmonary lobes on lung parenchyma windowed slices, the software estimated total lung volume (expressed as cubic centimeter [cc]) and also differentiated diverse areas of lung parenchyma based on Hounsfield Unit (HU) thresholds. To quantify and differentiate between normal and pathologic lung parenchyma, a –740-HU threshold was set: areas with higher-density values were considered pathologic (residual lung damage, expressed as percentage of the whole lung volume).48 One-year follow-up imaging was compared to chest CT scans of the same patients acquired 3 months after ICU discharge. Accordingly, any change in the burden of residual lung damage was noted.
Statistical Analysis
Statistical analysis was performed using Stata 16 (StataCorp LP, College Station, TX). Data were presented as medians with interquartile range (25th-75th percentiles) or as mean with SD. Mean and SD were used with normally distributed variables, whereas median and interquartile range were used with nonnormally distributed variables. Categorical and dichotomous variables were presented as absolute number and percentages (%). No data imputation for missing data was performed.
The authors compared questionnaire data obtained at 2 months and CT scan at 3 months with the same data collected at 1 year. Dichotomous variables were compared using chi-square test or Fisher exact test, while continuous variables were compared using Student t test or Wilcoxon sum-rank test as appropriate.
The authors compared baseline characteristics and treatment data of patients with a good overall recovery (defined as Glasgow Outcome Scale extended of 7 or greater) with patients with persistent disability. Data were compared between the groups using Fisher exact tests or Wilcoxon rank-sum tests. The authors included variables with a p value of less than 0.05 at univariate analysis in a multiple logistic regression model to predict good 1-year functional outcome. Collinearity and overfitting were assessed using a stepwise regression model and Pearson correlation test. In the multiple logistic regression analyses, the authors expressed clinical factors or potential confounding variables as odds ratio (OR), with 95% CI. A p value < 0.05 was considered significant.
A convenience sample was chosen for this preliminary observational study. A post hoc power calculation considering a 1-year post–ICU-discharge mortality for patients with ARDS of 10%8 and an alpha of 0.05 showed that the study had a power of 80.5%.
Results
Baseline Characteristics of Patients and ICU Course
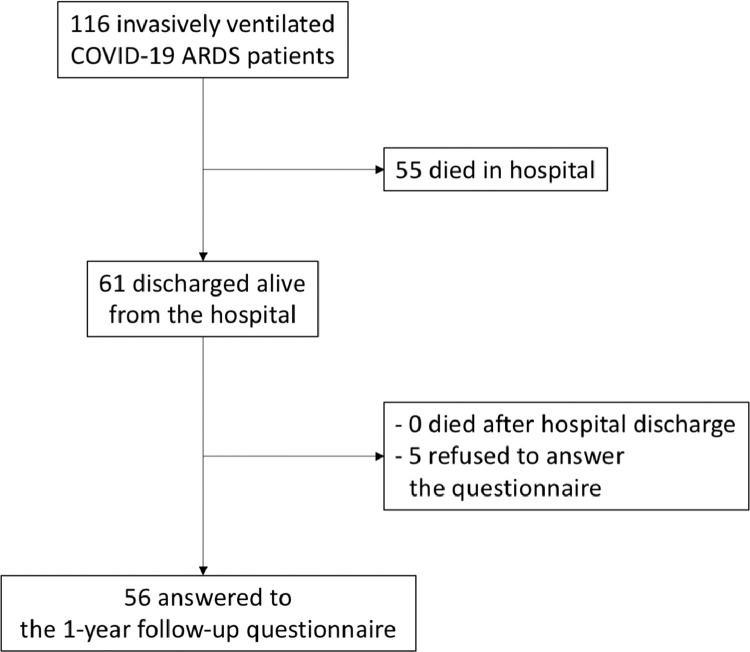
Overall, a total of 116 patients with COVID-19–related ARDS were admitted to an ICU at the authors’ institution and received invasive mechanical ventilation (Fig 1 ). Of these, 61 patients (52.6%) were discharged alive from the hospital. No patient was lost to 1-year follow-up and all patients discharged from the hospital were alive (1-year survival rate 61/61 [100%]). Five patients refused to respond to a 1-year follow-up questionnaire (questionnaire response rate 56/61 [91.8%]) (Fig 1).
Fig. 1.
Flow diagram showing the screening to follow-up.
Detailed baseline characteristics and general ICU course of the 56 patients included in the study are presented in Table 1 , while data on specific medical therapies received are presented in Supplementary Table 1. Patients were prevalently male (89%) and were 56 ± 11.9 years old.
Table 1.
Characteristics of the 56 Invasively Ventilated ICU Patients With COVID-19 ARDS Who Were Discharged Home and Replied to the 1-Year Follow-Up Questionnaire
| Baseline Characteristics | Value N: 56 | No. of Missing Values |
|---|---|---|
| Age (y), mean ± SD | 56 ± 11.9 | - |
| Male sex, no. (%) | 50 (89) | - |
| BMI (kg/m2), mean ± SD | 23.6 ± 10.4 | 10 |
| PaO2/FIO2 at ICU admission, mean ± SD (mmHg) | 120 ± 50.8 | 2 |
| ARDS severity at evaluation (according to Berlin criteria) | 2 | |
|
5 (9.3) | |
|
26 (48) | |
|
23 (43) | |
| Comorbidities | 6 | |
|
27 (48) | |
|
23 (41) | |
|
3 (5.4) | |
|
3 (5.4) | |
| Schooling | 22 | |
|
3 (8.8) | |
|
11 (32) | |
|
12 (35) | |
|
3 (8.8) | |
|
4 (12) | |
|
1 (2.9) | |
| Working status | 1 | |
|
31 (56) | |
|
24 (44) | |
| Smoking status | ||
|
36 (64) | |
|
3 (5.4) | |
|
17 (30) | |
| During ICU stay | ||
| CRRT, no. (%) | 9 (16) | - |
| Tracheostomy, no. (%) | 15 (27) | - |
| Prone positioning, no. (%) | 40 (72) | 3 |
| Inotropic support/vasopressors, no. (%) | 46 (87) | 3 |
| ECMO, no. (%) | 3 (5.4) | - |
| Neuromuscular blocking agents, no. (%) | 47 (89) | 3 |
| Days in hospital before ICU admission, median (IQR) | 3 (0-5) | 2 |
| Length of mechanical ventilation, median (IQR) | 11.5 (7-19) | 2 |
| Length of ICU stay, median (IQR) | 13 (9-21) | 2 |
| Length of overall hospital stay, median (IQR) | 30 (23-44) | 7 |
| Days from ICU discharge to follow-up, median (IQR) | 349 (343-356) | 2 |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; CRRT, continuous renal-replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; PaO2/FIO2, arterial partial pressure of oxygen to fraction of inspired oxygen ratio; SD, standard deviation.
The overall quality of life assessed through the EQ-5D-3L test showed that the vast majority of patients had no difficulty in walking (82%), self-care (95%), or usual activities (84%). A total of 5 patients (8.9%) reported severe anxiety/depression (Table 2 ).
Table 2.
Quality of Life, Psychological, Cognitive, and Miscellaneous Outcomes of 56 Invasively Ventilated ICU Patients With COVID-19 ARDS: 1-Year Follow-Up
| Items | Value N: 56 | No. of Missing Values |
|---|---|---|
| EuroQol 5 Dimensions 3 Levels (EQ5D3L)—mobility | - | |
|
46 (82) | |
|
1 (1.8) | |
|
7 (13) | |
|
2 (3.6) | |
| EuroQol 5 Dimensions 3 Levels (EQ5D3L)—self-care | - | |
|
53 (95) | |
|
1 (1.8) | |
|
2 (3.6) | |
| EuroQol 5 Dimensions 3 Levels (EQ5D3L)—usual activities | - | |
|
47 (84) | |
|
1 (1.8) | |
|
6 (11) | |
|
2 (3.6) | |
| EuroQol 5 Dimensions 3 Levels (EQ5D3L)—pain or discomfort | - | |
|
34 (61) | |
|
2 (3.6) | |
|
17 (30) | |
|
3 (5.4) | |
| EuroQol 5 Dimensions 3 Levels (EQ5D3L)—anxiety and depression | - | |
|
36 (64) | |
|
15 (27) | |
|
5 (8.9) | |
| Visual analog scale (VAS) for self-perceived health state, mean ± SD | 76 ± 18.7 | - |
| Hospital Anxiety and Depression Scale (HADS)—anxiety, median (IQR) | 3 (1-6) | 2 |
| Hospital Anxiety and Depression Scale (HADS)—depression, median (IQR) | 2 (0-5) | 2 |
| Post-Traumatic Stress Disorder Checklist for DSM-5 (PCL-5), median (IQR) | 7 (2-15) | 2 |
| Insomnia Severity Index (ISI), median (IQR) | 0 (0-4) | 1 |
| Italian telephone Mini Mental State Examination (I-tel MMSE), median (IQR) | 22 (22-22) | 1 |
| Working status | 1 | |
|
31 (56) | |
|
18 (33) | |
|
- | |
|
6 (11) | |
| Alteration in smell before ICU, no. (%) | 5 (8.9) | |
| Persisting after 1 year, no. (%) | 4 (7.1) | |
| Alteration in taste before ICU, no. (%) | 5 (8.9) | |
| Persisting after 1 year, no. (%) | 4 (7.1) | |
| Discrimination owing to the disease—personal—at least 1 episode, no (%) | 4 (7.1) | |
| None, no. (%) | 52 (93) | |
| Discrimination owing to the disease—family—at least 1 episode, no. (%) | 4 (7.1) | |
| None, no. (%) | 52 (93) | |
| Denied access to non-urgent care, no. (%) | 3 (5.4) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
At 1-year follow-up, psychological tests confirmed low rates of anxiety, depression, PTSD, insomnia, and cognitive impairment (Table 2).
Six patients (11% of the total number and 16% of those able to work at baseline) reported not being able to work due to a disease-dependent reason (Table 2).
A total of 45 of 56 patients (80.3%) reported good recovery as defined by the GOSe. Similarly, 93% of patients had no dyspnea at rest, while 16 of 56 (28.5%) reported exertional dyspnea (ranging from “light” to “very strong”). A total of 50 patients (89%) reported walking independently anywhere. The MNA-SF showed that 19 of 56 patients (33.9%) were malnourished or at risk for malnutrition (Table 3 ).
Table 3.
Functional Outcomes of 56 Invasively Ventilated ICU Patients With COVID-19 ARDS: 1-Year Follow-Up
| Items | Value N: 56 | No. of Missing Value |
|---|---|---|
| Glasgow Outcome Scale extended (GOSe) | - | |
|
36 (64) | |
|
9 (16) | |
|
6 (11) | |
|
2 (3.6) | |
|
1 (1.8) | |
|
2 (3.6) | |
| Dyspnea at rest (Borg Category Ratio 10 scale) | ||
|
52 (93) | |
|
1 (1.8) | |
|
1 (1.8) | |
|
1 (1.8) | |
|
1 (1.8) | |
| Exertional dyspnea (Borg Category Ratio 10 scale) | - | |
|
30 (54) | |
|
8 (14) | |
|
2 (3.6) | |
|
8 (14) | |
|
2 (3.6) | |
|
1(1.8) | |
|
2 (3.6) | |
|
1 (1.8) | |
|
2 (3.6) | |
| Mini Nutritional Assessment–Short Form (MNA-SF) | - | |
|
5 (8.9) | |
|
14 (24) | |
|
37 (67) | |
| Functional Ambulation Classification (FAC) | ||
|
50 (89) | |
|
2 (3.6) | |
|
2 (3.6) | |
|
1 (1.8) | |
|
1 (1.8) |
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Episodes of reported personal or family discrimination due to their COVID-19 illness were reported by 4 (7.1%) and 4 (7.1%) patients, respectively (Table 2).
Alterations in smell and taste were present before ICU admission in 5 (8.9%) and 5 (8.9%) patients, respectively, and persisted after hospital discharge in 4 (7.1%) and 4 (7.1%) patients (Table 2).
Comparison between questionnaire results obtained at 2-month and 1-year follow-ups are presented in Supplementary Tables 2 and 3. A total of 35 patients responded to both follow-ups and were included in this analysis. Overall, the authors observed the most significant improvements in the areas of working status, exertional dyspnea, nutrition status, and recovery according to GOSe. On the contrary, the authors observed limited improvement in quality of life as assessed by the EQ-5D-3L questionnaire, in cognitive and psychological outcomes, and in the functional ambulation classification.
Overall recovery expressed as GOSe was not different between those who were hospitalized for less than 2 months (N = 41) and those hospitalized longer than 2 months (N = 13) (median GOSe 8 [7-8] v 8 [7-8], respectively, p = 0.61, with data available for 54 patients).
Chest CT Scans
A total of 36 patients (64.3%) underwent 1-year follow-up chest CT scan. Of these, 29 of 36 (80.5%) already had a previous follow-up chest CT scan 3 months after ICU discharge, and were enrolled subsequently in this analysis. Three months after ICU discharge, 8 patients (27.6%) had a fibrotic-like CT pattern; at 1-year follow-up, 4 patients only (13.8%) kept fibrotic-like changes. Quantitative CT features are summarized in Table 4 . At 1-year follow-up, the median residual lung damage was 7.6% (5.6-10.7) versus 17.3% (12.1-23.1) 3 months after ICU discharge. Between these 2 points, total lung volume demonstrated a median increase of 925 cc (318-1194) (Supplementary Table 4).
Table 4.
Quantitative Assessment of Residual Lung Damage and Total Lung Volume at Both Points (3-Month and 1-Year Follow-Up)
| Residual Disease Burden (%) | |||
|---|---|---|---|
| 3-Month Follow-Up | 1-Year Follow-Up | Median Decrease | |
| Both lungs | 17 | 7.6 | 9.9 |
| (12.1-23.1) | (5.5-10.7) | (4.9-15.1) | |
| Right lung | 18 | 7.9 | 9.8 |
| (11.5-21. 7) | (5.5-9.8) | (3.6-14.2) | |
| Left lung | 18 | 8.2 | 10.4 |
| (10.3-28.6) | (5.5-11.8) | (3.1-15.3) | |
| Right upper lobe | 15 | 6.9 | 8 |
| (10.3-21.9) | (5.2-10.1) | (3.9-14.8) | |
| Right middle lobe | 8.1 | 4.5 | 3.8 |
| (5.3-16.3) | (2.7-8.5) | (1-7.22) | |
| Right lower lobe | 21 | 8.6 | 10.1 |
| (12.8-27.1) | (5.7 – 12.6) | (5.7-17.9) | |
| Left upper lobe | 13.7 | 6.6 | 7.0 |
| (9.5-20.8) | (4.7-11.6) | (3.1-11.8) | |
| Left lower lobe | 23.1 | 8.5 | 12.5 |
| (10.7-30.4) | (5.9-13.4) | (4.1-18.1) | |
An example of chest CT findings over time is presented in Supplementary Figure 2.
Predictors of Good Overall Recovery at 1 Year
Results of univariate and multiple logistic regression analyses to assess predictors of good 1-year overall recovery are presented in the Supplementary Tables 5 and 6. Multiple logistic regression analysis identified having few comorbidities as the only independent predictor of good overall 1-year recovery (OR = 0.18; 95% CI = 0.04 to 0.78; p = 0.023).
Discussion
Key Findings
In this observational study, the authors found that 1-year mortality for COVID-19–related ARDS requiring IMV was low in patients who survived the initial hospitalization. In addition, the authors found that, at 1-year follow-up, most patients were free of symptoms. Finally, follow-up chest CT scans showed significant, progressive regression of residual lung damage, with low prevalence of pulmonary fibrotic-like changes.
Relationship to Previous Studies
Previous studies performed on patients with COVID-19 generally reported follow-up limited to 4-to-6 months after critical illness,18 , 19 , 49, 50, 51, 52 with limited long-term data available. A small study including 23 patients reported a 1-year survival rate of 57%, consistent with these findings.17
Collectively, follow-up studies investigating short-term recovery after critical COVID-19 showed a significant burden of functional limitations and psychological sequelae in ICU survivors, but with gradual improvement over the period from discharge to 6 months.50 , 52
In the largest study, Morin et al. assessed 4-month respiratory, cognitive, and functional outcomes in 478 patients with COVID-19, including 142 who had been admitted to the ICU and 73 intubated patients.19 In their study, the authors used different scales but dyspnea was reported in 34% of intubated patients, somewhat higher than the 28% of exertional dyspnea. Similarly to this study, anxiety or depression was present in about 25% of patients, and fibrotic changes were observed in 20% of patients. Unfortunately, the authors did not present information on post-ICU discharge survival, although the overall mortality after hospitalization was 12% in their cohort. Other studies showed a relatively low postdischarge mortality, similar to this study, with variable rates of radiologic, functional, and psychological recovery.18 , 50 , 51
Interestingly, in contrast to previous publications,8 , 51 the authors found that age, duration of ICU stay and mechanical ventilation were not associated independently with 1-year outcome, while they identified baseline comorbidities as a key risk factor. However, these findings simply may have been due to different definitions of good outcome and different lengths of follow-up.
However, heterogeneity of previous studies in enrolled patient population (hospitalized/nonhospitalized, critically ill/noncritically ill, ventilated/nonventilated patients), the different point of the follow-up (most studies had shorter follow-up), and the different metrics adopted by researchers to assess the outcomes makes these findings only partially comparable with previous literature.
Comparison with ARDS not related to COVID-19 is difficult due to frequent use of different scales to assess quality of life and recovery. Interestingly, the authors observed a higher postdischarge survival compared with ARDS not related to COVID-19, in whom post-ICU mortality has been reported to be as high as 11%.8
However, available studies collectively showed reduced quality of life, functional recovery, and several psychological sequelae, with a relatively high prevalence of PTSD.5 , 7 , 53, 54, 55 The data, together with data from other studies, suggest that recovery from critical COVID-19–related ARDS is somewhat better than for ARDS not related to COVID-19, although studies providing direct comparison between these 2 populations are lacking.
Interestingly, on that note, the authors observed a median residual lung damage at 1-year follow-up of 7.6%, slightly lower compared with previous reports concerning ARDS not related to COVID-19, reporting a residual lung damage involving 10% to 25% of lung parenchyma.56
Implications of Study Findings
This study, together with data from literature, suggested that, in patients with COVID-19–related ARDS, mortality after ICU discharge is low and overall long-term recovery is good. In particular, clinical findings were consistent with radiologic findings obtained through follow-up chest CT scans. Therefore, the data underlined that clinicians caring for patients with COVID-19, and in particular for those admitted to an ICU, should be reassured that patients surviving the acute phase will have a good functional recovery after 1 year. Of course, the data should be interpreted considering that ICU mortality of patients with COVID-19–related ARDS is approximately 50%.15 Furthermore, this study suggested that post-ICU multidisciplinary follow-up for critically ill patients may have an important role for long-term outcomes.14 , 57
The Centers for Disease Control and Prevention, together with the United States Department of Human Health Services, raised attention over the post–COVID-19 conditions, now classified as a disease according to the Americans with Disabilities Act.58 This postviral syndrome is defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms.”59 Inside this syndrome, 2 further categories exist: subacute or ongoing symptomatic COVID-19 (between weeks 4 and 12 beyond acute infection), and chronic or post–COVID-19 syndrome (beyond 12 weeks from acute infection). Several hypotheses have been proposed to explain the long–COVID-19, and as well as for the postintensive care syndrome, as its pathophysiology probably is multifactorial: an excessive inflammatory response, the persistence of the virus in certain reservoir tissues, the immune dysregulation leading to reactivation of pathogens, the role of the host microbiome, coagulation and clotting abnormalities, autoimmunity, and a direct role of the virus.60 Inside this complex disease, the authors found a relatively low prevalence of neurologic impairment at 1 year compared with previous literature and studies with shorter follow-up.61 Indeed, among COVID-19 survivors, anxiety, depression, sleep disturbance, and PTSD were reported in up to 30% to 40%,59 although the prevalence of these disturbances was highly variable across studies.62 It is possible that the relatively young age of the patients and the prevalence of men63 led to better recovery from critical illness. Furthermore, symptoms of postintensive care syndrome have been shown to improve over time,14 and it is possible that the authors’ dedicated post–COVID-19 follow-up clinic helped in subsequent recovery.57 , 64 , 65 Notably, in a 6-month postdischarge assessment of patients with COVID-19, women, compared to men, were found to have an OR 2.22 (95% CI 1.24-3.89) for pulmonary diffusion impairment, an OR 1.8 (CI 1.39-2.34) for anxiety and depression, and an OR 1.33 (CI 1.05-1.67) for fatigue and muscle weakness.63 Indeed, female sex was confirmed in several publications to be a risk factor for long-term psychological/psychiatric sequalae,62 and several studies also confirmed improvement of symptoms over time after COVID-19–related ARDS.62
Notably, the data were collected at the beginning of the COVID-19 pandemic, thus, before the delta variant became prevalent. In particular, the first case of delta variant in Italy was registered in April 2021, 1 year after admission in the ICU of the last patient. The data, therefore, may not apply to patients with the delta variant.
This study confirmed radiologic data from previous studies, which reported an unexpected low prevalence of fibrotic-like changes at short-term follow-up,19 , 50 , 66 and also provided new insights suggesting a reversal of these changes and further lung healing after 1 year. A possible explanation could lie in the previously proposed virus-induced lung frailty, which is thought to be a main contributor to the high incidence of barotrauma in patients with COVID-19.67 , 68 In this prospective, the honeycombing CT pattern, when present, could represent a subpleural cluster of small pneumatoceles rather than cystic air spaces resulting from irreversible lobular disruption. Furthermore, residual lung damage does not correspond necessarily to lung fibrosis, which instead has a strict radiologic definition.47
Finally, a relevant proportion of the patients received hydroxychloroquine as adjuvant antiviral therapy. Notably, following publication of several randomized trials showing negative results,69 use of hydroxychloroquine as treatment for COVID-19 greatly decreased and no longer is recommended. Although the authors cannot exclude that use of the drug contributed to the good long-term outcome, this seems unlikely given the negative results of major randomized trials on its use.
Strengths and Limitations
This was the first study presenting clinical and radiologic 1-year follow-up data for patients with COVID-19–related ARDS. A strength of this study was the absence of patients lost to follow-up and the almost complete questionnaire follow-up. Furthermore, the authors employed several previously validated scales to assess functional, psychological, and cognitive outcomes. Finally, the presence of quantitative radiologic data represented a major strength of the present study.
The study was limited by its single-center design, the small sample size, and the absence of a non-COVID-19–related ARDS control group and noninvasively ventilated patients with COVID-19. However, obtaining a comparable control group during the same time was almost impossible due to the overwhelming number of COVID-19 cases. The present study focused only on patients who received IMV (ie, the most severely ill patients), while the long-term outcome of patients treated with noninvasive ventilation currently is under investigation.24 , 70 , 71
Patients included in this study were relatively young and prevalently male, with normal body mass index and few comorbidities. Older patients with greater burden of comorbidities were less likely to be admitted to the ICU during the pandemic crisis24 , 71 and had higher risk of death. Therefore, the data may suffer from selection, sex, and survival bias.3 , 15 However, the age range of patients admitted to the authors’ ICUs was comparable to that of patients admitted to ICUs in the whole Lombardy region during the same period, 3 , 15 and a male prevalence of ICU and hospitalized patients already was known.72
The authors did not collect data on long-term occurrence rate of cardiovascular and thrombotic events, or long-term organ function (eg, renal function or pulmonary function tests). However, investigation of these outcomes was not among the objectives of this study and is currently the subject of separate investigations.73
The study was not randomized, so the authors cannot determine whether referral of patients to the post–COVID-19 follow-up clinic57 has influenced long-term outcome.
Future Studies and Prospects
Future studies should confirm in a larger sample size the findings of a low postdischarge mortality and good functional and radiologic recovery following COVID-19–related ARDS. In addition, the role of post-ICU follow-up clinics, as well as multidisciplinary long-term management protocols, should be assessed and defined.
Finally, future studies should define further the correlation among pathogenesis of COVID-19 lung damage, acute-phase treatments, and long-term lung fibrosis.
Conclusion
This study suggested that, among ICU patients with COVID-19–related ARDS who required invasive mechanical ventilation and were discharged alive from the hospital (approximately 50% of ICU patients with COVID-19–related ARDS receiving invasive ventilation), 1-year survival is high. Furthermore, overall recovery and quality of life of survivors are good after 1 year. Radiologic findings were consistent with clinical findings, showing a progressive reduction in residual lung damage. Recovery seems better than for ARDS not related to COVID-19, although a direct comparison is lacking.
Conflict of Interest
None.
Acknowledgments
The authors thank all the personnel of San Raffaele Hospital for the dedication to these patients and for the support in data collection.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2021.11.032.
Appendix. Supplementary materials
References
- 1.Fagiuoli S, Lorini FL, Remuzzi G. Adaptations and lessons in the province of Bergamo. N Engl J Med. 2020;382:e71. doi: 10.1056/NEJMc2011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zangrillo A, Beretta L, Silvani P, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: Facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22:91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bein T, Weber-Carstens S, Apfelbacher C. Long-term outcome after the acute respiratory distress syndrome: Different from general critical illness? Curr Opin Crit Care. 2018;24:35–40. doi: 10.1097/MCC.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42:725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 8.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2694. jrm00063. [DOI] [PubMed] [Google Scholar]
- 10.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson CL, Udy AA, Bailey M, et al. The impact of disability in survivors of critical illness. Intensive Care Med. 2017;43:992–1001. doi: 10.1007/s00134-017-4830-0. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Vincent JL, Angus DC, et al. Recovery after critical illness: Putting the puzzle together–a consensus of 29. Crit Care. 2017;21:296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: A multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leggieri C, Dezza L, Oltolini B, et al. Long-term quality of life after intensive care unit admission (a single-center observational study) Gen Reanimatol. 2021;17:72–87. [Google Scholar]
- 15.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasulo FA, Piva S, Latronico N. Long-term complications of COVID-19 in ICU survivors: What do we know? [e-pub ahead of print]. Minerva Anestesiol. doi: 10.23736/S0375-9393.21.16032-8. [DOI] [PubMed]
- 17.Rajajee V, Fung CM-C, Seagly KS, et al. One-year functional, cognitive, and psychological outcomes following the use of extracorporeal membrane oxygenation in coronavirus disease 2019: A prospective study. Crit Care Explor. 2021;3:e0537. doi: 10.1097/CCE.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Writing Committee for the COMEBAC Study Group. Morin L, Savale L, Pham T, et al. Writing Committee for the COMEBAC Study Group Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monti G, Leggieri C, Fominskiy E, et al. Two months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol Scand. 2021;65:912–920. doi: 10.1111/aas.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zangrillo A, Beretta L, Scandroglio AM, et al. COVID-BioB Study Group Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovere-Querini P, Tresoldi C, Conte C, et al. Biobanking for COVID-19 research. Panminerva Med. doi: 10.23736/S0031-0808.20.04168-3. In press. [DOI] [PubMed]
- 23.Palumbo D, Campochiaro C, Belletti A, et al. COVID-BioB Study Group Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: Differences between first and second Italian pandemic wave. Eur J Intern Med. 2021;88:144–146. doi: 10.1016/j.ejim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez GA, Bozzolo EP, Castelli E, et al. Continuous positive airway pressure and pronation outside the intensive care unit in COVID 19 ARDS . Minerva Med. doi: 10.23736/S0026-4806.20.06952-9. In press. [DOI] [PubMed]
- 25.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morselli F, Vitali G, Brioschi E, et al. Feasibility and safety of angiotensin II administration in general ward patients during COVID-19 pandemic: A case series. Crit Care Resusc. 2020;22:388–390. doi: 10.51893/2020.4.RL1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 28.Vignon P, Evrard B, Asfar P, et al. Fluid administration and monitoring in ARDS: Which management? Intensive Care Med. 2020;46:2252–2264. doi: 10.1007/s00134-020-06310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhazzani W, Belley-Cote E, Møller MH, et al. Neuromuscular blockade in patients with ARDS: A rapid practice guideline. Intensive Care Med. 2020;46:1977–1986. doi: 10.1007/s00134-020-06227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foti G, Giannini A, Bottino N, et al. Management of critically ill patients with COVID-19: Suggestions and instructions from the coordination of intensive care units of Lombardy. Minerva Anestesiol. 2020;86:1234–1245. doi: 10.23736/S0375-9393.20.14762-X. [DOI] [PubMed] [Google Scholar]
- 31.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JTL, Pettigrew LEL, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J Neurotrauma. 1998;15:573–580. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 33.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holden M, Gill K, Magliozzi M, et al. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther. 1984;64:35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 35.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 36.Morishita S, Yamauchi S, Fujisawa C, Domen K. Rating of perceived exertion for quantification of the intensity of resistance exercise. Int J Phys Med Rehabil. 2013;1:172. [Google Scholar]
- 37.Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J Nutr Heal Aging. 2009;13:782–788. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 38.EuroQol – A new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 39.Scalone L, Cortesi PA, Ciampichini R, et al. Italian population-based values of EQ-5D health states. Value Health. 2013;16:814–822. doi: 10.1016/j.jval.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Jutte JE, Needham DM, Pfoh ER, et al. Psychometric evaluation of the Hospital Anxiety and Depression Scale 3 months after acute lung injury. J Crit Care. 2015;30:793–798. doi: 10.1016/j.jcrc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 42.Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. J Trauma Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 43.Ashbaugh AR, Houle-Johnson S, Herbert C, et al. Psychometric validation of the English and French versions of the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) PLoS One. 2016;11 doi: 10.1371/journal.pone.0161645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosendahl J, Kisyova H, Gawlytta R, et al. Comparative validation of three screening instruments for posttraumatic stress disorder after intensive care. J Crit Care. 2019;53:149–154. doi: 10.1016/j.jcrc.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metitieri T, Geroldi C, Pezzini A, et al. The ITEL-MMSE: An Italian telephone version of the Mini-Mental State Examination. Int J Geriatr Psychiatry. 2001;16:166–167. doi: 10.1002/1099-1166(200102)16:2<166::aid-gps290>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 47.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 48.De Cobelli F, Palumbo D, Ciceri F, et al. Pulmonary vascular thrombosis in COVID-19 pneumonia. J Cardiothorac Vasc Anesth. 2021;35:3631–3641. doi: 10.1053/j.jvca.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Graaf MA, Antoni ML, ter Kuile MM, et al. Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker AJ, Humbir A, Tiwary P, et al. Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth. 2021;126:e217–e219. doi: 10.1016/j.bja.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamberini L, Mazzoli CA, Sintonen H, et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res. 2021;30:2805–2817. doi: 10.1007/s11136-021-02865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musheyev B, Borg L, Janowicz R, et al. Functional status of mechanically ventilated COVID-19 survivors at ICU and hospital discharge. J Intensive Care. 2021;9:31. doi: 10.1186/s40560-021-00542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marti J, Hall P, Hamilton P, et al. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): Secondary analysis of a randomised controlled trial. J Intensive Care. 2016;4:56. doi: 10.1186/s40560-016-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biehl M, Kashyap R, Ahmed AH, et al. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: A population-based study. Crit Care. 2015;19:356. doi: 10.1186/s13054-015-1062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: A 2-year longitudinal study. Crit Care Med. 2015;43:642–653. doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiumello D, Coppola S, Froio S, et al. What's next after ARDS: Long-term outcomes. Respir Care. 2016;61:689–699. doi: 10.4187/respcare.04644. [DOI] [PubMed] [Google Scholar]
- 57.Rovere-Querini P, De Lorenzo R, Conte C, et al. Post-COVID-19 follow-up clinic: Depicting chronicity of a new disease. Acta Biomed. 2020;91(9-S):22–28. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guidance on “long COVID” as a disability under the ADA. HHS.gov. Available at: https://www.hhs.gov/civil-rights/for-providers/civil-rights-covid19/guidance-long-covid-disability/index.html#footnote8_y2ge3sd. Accessed October 9, 2021.
- 59.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortona E, Malorni W., Long COVID. To investigate immunological mechanisms and sex/gender related aspects as fundamental steps for a tailored therapy. Eur Respir J. 2021 doi: 10.1183/13993003.02245-2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maley JH, Brewster I, Mayoral I, et al. Resilience in survivors of critical illness in the context of the survivors’ experience and recovery. Ann Am Thorac Soc. 2016;13:1351–1360. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schou TM, Joca S, Wegener G, et al. Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review. Brain Behav Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Lorenzo R, Conte C, Lanzani C, et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Lorenzo R, Cinel E, Cilla M, et al. Physical and psychological sequelae at three months after acute illness in COVID-19 survivors . Panminerva Med. doi: 10.23736/S0031-0808.21.04399-8. In press. [DOI] [PubMed]
- 66.van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belletti A, Todaro G, Valsecchi G, et al. Barotrauma in coronavirus disease 2019 patients undergoing invasive mechanical ventilation: A systematic literature review. Crit Care Med. doi: 10.1097/CCM.0000000000005283. In press. [DOI] [PMC free article] [PubMed]
- 68.Belletti A, Palumbo D, Zangrillo A, et al. COVID-BioB Study Group Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth. 2021;35:3642–3651. doi: 10.1053/j.jvca.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horby Peter, Mafham Marion, Linsell Louise, et al. RECOVERY Collaborative Group Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Damanti S, Ramirez GA, Bozzolo EP, et al. 6-month respiratory outcomes and exercise capacity of COVID-19 acute respiratory failure patients treated with CPAP. Intern Med J. 2021;51:1810–1815. doi: 10.1111/imj.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramirez GA, Bozzolo EP, Gobbi A, et al. Outcomes of non-invasive ventilation as the ceiling of treatment in patients with COVID-19. Panminerva Med. doi: 10.23736/S0031-0808.21.04280-4. In press. [DOI] [PubMed]
- 72.Baiardo Redaelli M, Landoni G, Di Napoli D, et al. Novel coronavirus disease (COVID-19) in Italian patients: Gender differences in presentation and severity. Saudi J Med Med Sci. 2021;9:59–62. doi: 10.4103/sjmms.sjmms_542_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Compagnone N, Palumbo D, Cremona G, et al. Residual lung damage following ARDS in COVID-19 ICU survivors. Acta Anaesthesiol Scand. doi: 10.1111/aas.13996. In press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.