Figure 1.
Isolation and characterization of Beta SARS-CoV-2-specific mAbs
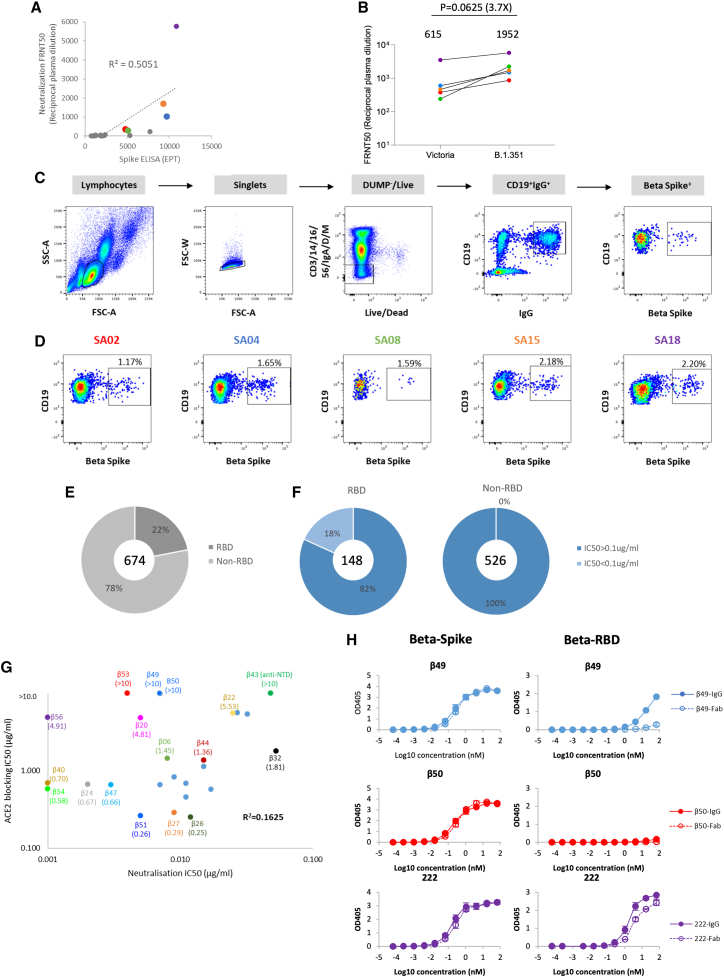
(A) Comparison of Beta SARS-CoV-2 neutralization and S binding ELISA by convalescent plasma from confirmed Beta SARS-CoV-2 infected donors. Plasma samples with FRNT50 >1:250 are highlighted and correspond to the cases shown in (D).
(B) Neutralization titers against SARS-CoV-2 strain Victoria and the Beta variant for the 5 selected plasma samples with potent neutralizing properties, analysis used the Wilcoxon matched-pairs signed rank test and two-tailed p values were calculated; geometric means are indicated above each column.
(C) Schematic of the Beta SARS-CoV-2 mAb isolation strategy.
(D) Antigen-specific single B cells were isolated using labeled recombinant S protein as bait. The frequency of S-reactive IgG+ B cells was measured by FACS.
(E) Epitope mapping of Beta SARS-CoV-2 specific mAbs against S and RBD were evaluated by ELISA.
(F) Neutralization potencies (IC50) between anti-S (non-RBD) and anti-RBD mAbs against authentic Beta SARS-CoV-2 using a FRNT50 test.
(G) Comparison of IC50 values for ACE2 binding and FRNT50 titers for the 27 potent mAbs, those selected for further structural study are highlighted.
(H) Binding of Beta-49 and -50 Fab and IgG1 to Beta S trimer or Beta RBD measured by ELISA, comparison is made with binding of mAb 222, data are shown as mean ± SEM. See also Table S1A.