Abstract
Background
Preliminary analysis from the Vax-On study did not find a correlation between cancer treatment type and antibody response to COVID-19 vaccination. We carried out a secondary subgroup analysis to verify the effects of comprehensive cancer treatment classification on vaccine immunogenicity.
Methods
The Vax-On study prospectively enrolled patients who started a two-dose messenger RNA-BNT162b2 vaccine schedule from 9 March 2021 to 12 April 2021 (timepoint-1). Those on active treatment within the previous 28 days accounted for the exposed cases. Patients who had discontinued such treatment by at least 28 days or received intravesical therapy represented the control cases. Quantification of immunoglobulin G (IgG) antibodies against the receptor binding domain of the S1 subunit of the SARS-CoV-2 spike protein was carried out before the second dose (timepoint-2) and 8 weeks thereafter (timepoint-3). Seroconversion response was defined at ≥50 arbitrary units/ml IgG titer. Classification of antineoplastic agents was based on their pharmacodynamic properties.
Results
Three hundred and sixty-six patients were enrolled (86 and 260 as control and exposed cases, respectively). Univariate analysis revealed a significantly lower IgG titer after both doses of vaccine in subgroups treated with tyrosine kinase inhibitors (TKIs), multiple cytotoxic agents, alkylating agents, and topoisomerase inhibitors. At timepoint-3, seroconversion response was significantly impaired in the topoisomerase inhibitors and mechanistic target of rapamycin (mTOR) inhibitors subgroups. After multivariate testing, treatment with alkylating agents and TKIs was significantly associated with a reduced change in IgG titer at timepoint-2. Treatment with mTOR inhibitors resulted in a similar interaction at each timepoint. Cyclin-dependent kinase 4/6 inhibitor treatment was independently correlated with an incremental variation in IgG titer at timepoint-3. Specific subgroups (TKIs, antimetabolites, alkylating agents, and multiple-agent chemotherapy) predicted lack of seroconversion at timepoint-2, but their effect was not retained at timepoint-3. Eastern Cooperative Oncology Group performance status 2, immunosuppressive corticosteroid dosing, and granulocyte colony-stimulating factor use were independently linked to lower IgG titer after either dose of vaccine.
Conclusions
Drugs interfering with DNA synthesis, multiple-agent cytotoxic chemotherapy, TKIs, mTOR and cyclin-dependent kinase 4/6 inhibitors differentially modulate humoral response to messenger RNA-BNT162b2 vaccine.
Key words: COVID-19 vaccine, cancer, cytotoxic chemotherapy, targeted therapy, antibody response
Highlights
-
•
We analyzed the effects of comprehensive cancer treatment classification on mRNA-BNT162b2 vaccine immunogenicity.
-
•
Univariate analysis showed a humoral response impaired by polychemotherapy, DNA-damaging agents, TKIs, and mTOR inhibitors.
-
•
Multivariate analysis confirmed a negative interaction with alkylating agents, TKIs, and mTOR inhibitors.
-
•
ECOG PS2, corticosteroid therapy, and G-CSF use were independently associated with reduced humoral response.
-
•
Further confirmation of these findings may be valuable in prioritizing high-risk patients for the third dose of vaccine.
Introduction
The rapid and pandemic spread of COVID-19, caused by the novel SARS-CoV-2, has comprehensively affected the management of cancer patients worldwide.1 The incidence of COVID-19 among patients with an active diagnosis of malignancy and those receiving immunocompromising cancer therapies is thought to be higher than in the general population.2,3 Following a diagnosis of COVID-19, advanced disease status and immunosuppressive cancer treatment resulted in an increased risk of major complications and death.4,5 Recent data revealed an alarming prevalence of severe disease and mortality rates among COVID-19 patients with cancer of 34% and 20%, respectively. The same ratios were even higher in European patients.6
Phase III trials of SARS-CoV-2 messenger RNA (mRNA)-BNT162b2 (tozinameran)7 and mRNA-1273 (elasomeran)8 vaccines showed a favorable safety profile and high efficacy, conferring 94%-95% protection against COVID-19 in recipients aged 16 years or older. These data granted emergency use authorization by regulatory agencies and leading medical oncology societies recommended patients with active cancer or recent or planned cancer treatment to be prioritized for COVID-19 vaccination. On this basis, national oncology facilities were commissioned to provide a COVID-19 mRNA vaccine to all cancer patients undergoing immunosuppressive drug treatment or within 6 months after such treatment.
The Vax-On study was carried out at our institution as part of introducing tozinameran vaccination in actively treated cancer patients. Our preliminary findings confirmed a favorable safety profile and suggested that proximity to cancer treatment might hamper the immune response to the first dose of vaccine. The second dose induced an exponential rise in antibody titer, overcoming the detrimental effect of treatment on the immune response. Although several studies have found a correlation between generic cytotoxic chemotherapy and a lower level of antibody response, our multivariate analysis did not support this finding.9 Herein, we provide the results of a preplanned analysis of the Vax-On study, which aimed to assess the impact of antineoplastic agents on tozinameran immunogenicity using a comprehensive classification of their pharmacodynamic properties.10
Methods
Study design and subjects
The Vax-On study is a single-center, prospective observational study approved by the referring Ethics Committee (Protocol number: 595/CE Lazio 1, 28 April 2021; clinical trial identifier: EudraCT Number 2021-002611-54). Eligibility requirements were: age ≥18 years, histological diagnosis of solid tumor, ongoing active cancer treatment or its completion within the previous 6 months, and initiation of the two-dose tozinameran vaccine program. Exclusion criteria comprised life expectancy <12 weeks, active concomitant hematological malignancy, documented COVID-19 infection at any time before enrollment, direct contact with a COVID-19-positive person, or symptoms strongly suspicious for COVID-19 infection within 14 days before enrollment. According to the international guidelines, a negative SARS-CoV-2 real-time RT-PCR swab or serologic test was not required for either vaccination or inclusion in the study. Adherence to vaccination was voluntary and carried out after the acquisition of institutional informed consent. All eligible participants gave a specific written informed consent for study proposal and procedures, but participation was not a prerequisite for receiving the COVID-19 vaccination. The procedures used in this study adhere to the tenets of the 1964 Declaration of Helsinki and its subsequent amendments.
Endpoints and assessments
The primary endpoints were dynamic changes in serum titer of immunoglobulin class G (IgG) antibodies against the receptor binding domain (RBD) of the S1 subunit of the SARS-CoV-2 spike protein (S1) and seroconversion rates after each vaccine dose. The participants were vaccinated with two 30 μg doses of tozinameran given intramuscularly 21 days apart. The first injection (timepoint-1) was given at least 24 h before the anticancer treatment. The second vaccine administration took place exactly 21 days later (timepoint-2) with no delays or changes to the planned treatment regimen. According to our preplanned analysis, patients who received at least one course of active anticancer treatment within the 28 days before enrollment accounted for subgroups of exposed cases. Antimicrotubule agents, topoisomerase inhibitors, antimetabolites, and alkylating agents were the subgroups of cytotoxic chemotherapy. In the case of combination regimens, the agent with the highest immunosuppressive potential defined the subgroup. An additional comparison subgroup consisted of patients who received a combination of at least two cytotoxic drugs. Hormonal therapy, immune checkpoint inhibitors, poly (ADP-ribose) polymerase inhibitors, tyrosine kinase inhibitors (TKIs), monoclonal antibodies, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, and mechanistic target of rapamycin (mTOR) inhibitors were the biological therapy subgroups. Those who discontinued such treatment at least 28 days before enrollment or who received nonsystemic (i.e. intravesical) therapy made up the control subgroup. The study cut-off date for interim analysis was 30 June 2021.
Serological test
Blood draws were collected using standard tubes and separated by immediate centrifugation. Serum specimens were analyzed at each drawing time using chemiluminescent microparticle immunoassay technology. The SARS-CoV-2 IgG II Quant assay on ARCHITECT i2000sr automated platform (Abbott Laboratories, Diagnostics Division, Sligo, Ireland) was used for quantitative detection of anti-RBD S1 IgG antibodies in human serum or plasma as per the manufacturer's instructions.11,12 Test results are reported as arbitrary units (AU)/ml with a cut-off ≥50 AU/ml considered positive.
Statistical analysis
The subgroups were compared using Pearson's chi-square test for categorical data and Mann–Whitney U test for continuous variables. The Wilcoxon rank-sum test for continuous variables and the McNemar test for categorical variables were applied for paired comparisons within the same subgroup. A multivariate analysis was carried out by fitting a generalized linear model on logarithmic (log) IgG titer and seroconversion response as a function of predefined independent covariates. These included the subgroups defined by specific anticancer treatment as described in the preceding text and factors that showed a significant interaction with immunogenicity parameters from previous multivariate assessment [sex, age, Eastern Cooperative Oncology Group performance status (ECOG PS), treatment setting, corticosteroid therapy (≥10 mg daily prednisone equivalents for at least 7 days during previous 28 days), and granulocyte colony-stimulating factor (G-CSF) therapy (administration of G-CSF during previous 28 days)]. All tests carried out were two-sided and a P value <0.05 was considered significant. SPSS software (IBM SPSS Statistics for Windows, Version 23.0; IBM Corp., Armonk, NY) was used for all statistical evaluations.
Results
Patient characteristics
A total of 366 consecutive patients were enrolled from 9 March 2021 through 12 April 2021. As part of the exposed case subgroup, 280 patients received active anticancer treatment, while the remaining 86 cases comprised the control subgroup. Serologic testing for IgG titer was carried out in 357 (98%) and 336 (92%) patients at timepoint-2 and timepoint-3, respectively. Reasons for missing assessment at each timepoint are shown in the patients’ flow diagram (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100350). Of note, two patients (0.5%) were diagnosed with COVID-19 infection after the first dose, whereas no cases were reported after the second dose. As expected, there was a higher frequency of clinical features considered adversely prognostic in the exposed cohort. Treatment settings widespread in the control cohort, such as adjuvant or neoadjuvant chemotherapy, might explain this imbalance. Baseline characteristics of patients enrolled are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100350. A list of the drugs used in the study according to pharmacodynamic properties is provided in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100350.
Univariate analysis of immunogenicity
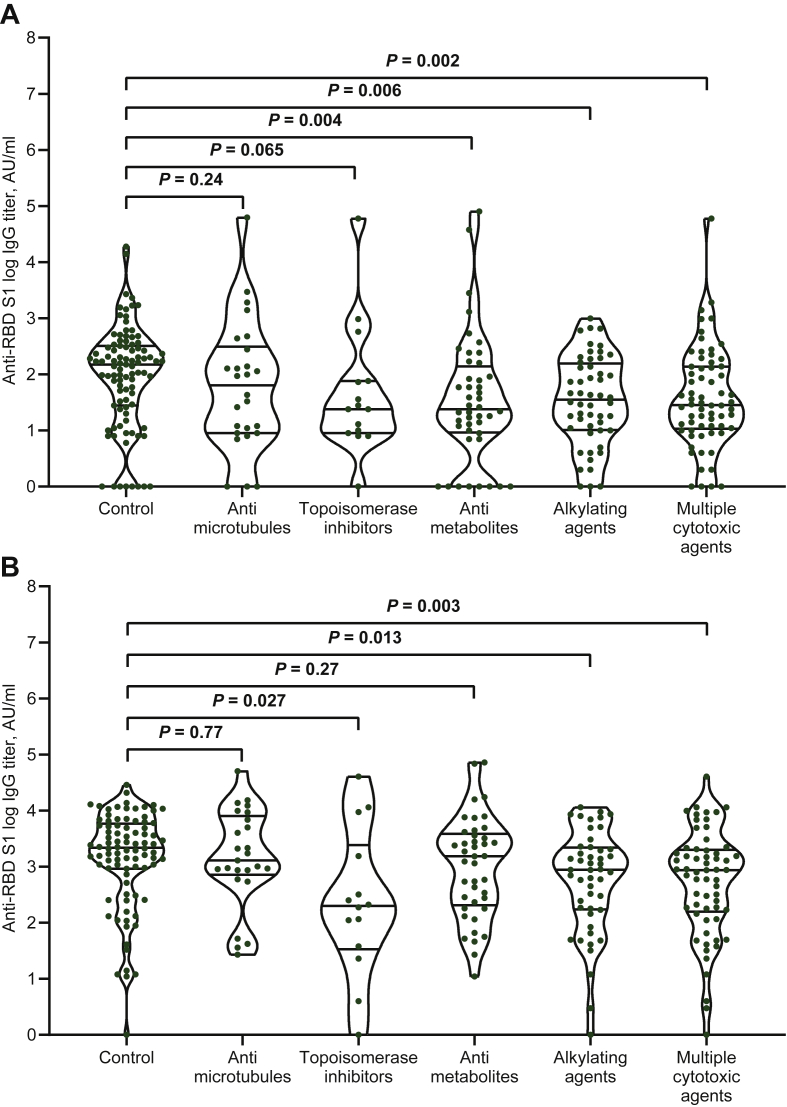
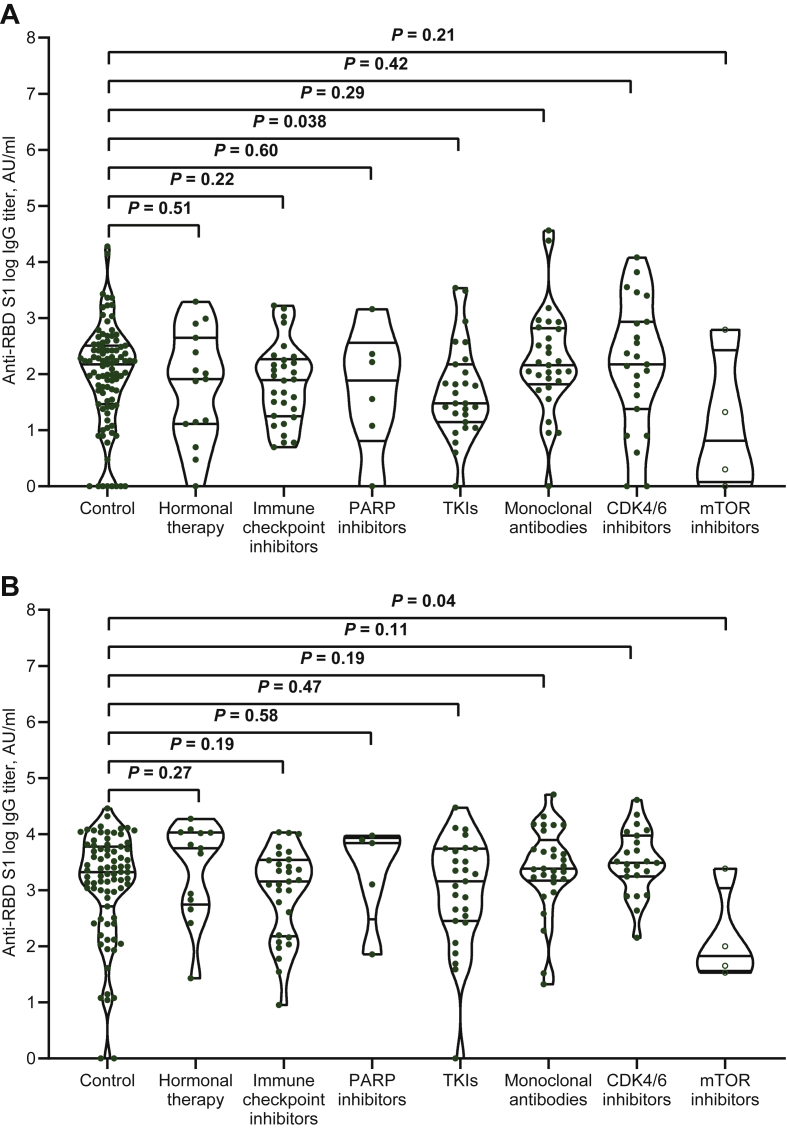
We first compared the variation in IgG titer and seroconversion rate between treatment subgroups and controls at each timepoint. Except for those treated with antimicrotubule agents, all cytotoxic chemotherapy subgroups had significantly lower median IgG titers and seroconversion rates at timepoint-2 (Table 1, Figure 1A). Patients receiving multiple cytotoxic agents, alkylating agents, and topoisomerase inhibitors also had lower IgG titers at timepoint-3 (Table 1, Figure 1B), but the seroconversion response was uniquely impaired in the last subgroup (Table 1). Patients treated with TKIs were significantly impaired in both vaccine efficacy parameters at timepoint-2 (Table 1, Figure 2A), whereas those treated with mTOR inhibitors had the same outcomes at timepoint-3 (Table 1, Figure 2B). Paired comparisons of timepoints-2 and -3 within the same subgroup revealed a significant increase in median IgG titer and seroconversion response in all patient subgroups except those treated with mTOR inhibitors (Table 1).
Table 1.
Comparison of antibody and seroconversion response of treatment subgroups versus control and within the same subgroup
| Treatment subgroup | Timepoint-2 (versus control) |
Timepoint-3 (versus control) |
Timepoint-3 versus timepoint-2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Evaluable, n (%) | Median IgG titer (IQR), AU/ml [P value] | Seroconversion ratea, n (%) [P value] | Evaluable, n (%) | Median IgG titer (IQR), AU/ml [P value] | Seroconversion ratea, n (%) [P value] | Median IgG titer (P value) | Seroconversion rate (P value) | |
| Control (N = 86) | 86 (100) | 144 (26-318) | 60 (69.8) | 79 (91.8) | 2096 (513-6021) | 72 (91.1) | <0.001 | <0.001 |
| Cytotoxic therapies | ||||||||
| Antimicrotubule agents (N = 28) | 26 (92.9) | 68 (9-318) [0.23] | 13 (50) [0.06] | 25 (89.3) | 1288 (729-8133) [0.66] | 22 (88) [0.65] | <0.001 | 0.003 |
| Topoisomerase inhibitors (N = 17) | 15 (88.2) | 24 (9-76) [0.05] | 5 (33.3) [0.006] | 14 (82.4) | 199 (34-3520) [0.018] | 10 (71.4) [0.03] | 0.035 | 0.040 |
| Antimetabolites (N = 44) | 44 (100) | 24 (9-143) [0.003] | 17 (38.6) [<0.001] | 41 (93.2) | 1530 (207-3872) [0.20] | 38 (92.7) [0.75] | <0.001 | <0.001 |
| Alkylating agents (N = 52) | 51 (98) | 39 (11-163) [0.005] | 21 (41.2) [<0.001] | 47 (90.3) | 882 (179-2240) [0.003] | 42 (87.5) [0.51] | <0.001 | <0.001 |
| Multiple cytotoxic agents (N = 68) | 66 (97.1) | 28 (11-137) [0.002] | 24 (36.4) [<0.001] | 61 (89.7) | 866 (157-1998) [0.003] | 52 (85.2) [0.27] | <0.001 | <0.001 |
| Biological therapies | ||||||||
| Hormonal therapy (N = 15) | 15 (100) | 82 (13-444) [0.53] | 9 (60) [0.44] | 13 (86.7) | 5583 (565-10 726) [0.27] | 12 (92.3) [0.82] | 0.001 | 0.038 |
| Immune checkpoint inhibitors (N = 32) | 29 (90.6) | 78 (18-185) [0.22] | 16 (55.2) [0.15] | 29 (90 .6) | 1436 (151-3480) [0.19] | 28 (96.6) [0.32] | <0.001 | <0.001 |
| PARP inhibitors (N = 6) | 6 (100) | 100 (9-447) [0.58] | 3 (50) [0.31] | 5 (83.3) | 6905 (670-8634) [0.56] | 5 (100) [0.52] | 0.043 | <0.001 |
| TKIs (N = 29) | 28 (96.5) | 30 (12-138) [0.036] | 12 (42.9) [0.01] | 27 (93.1) | 1443 (284-5524) [0.47] | 24 (88.9) [0.72] | <0.001 | <0.001 |
| Monoclonal antibodies (N = 30) | 29 (96.6) | 144 (66-658) [0.30] | 24 (82.8) [0.17] | 29 (96.6) | 2419 (1487-8466) [0.19] | 27 (93.1) [0.74] | <0.001 | 0.018 |
| CDK4/6 inhibitors (N = 23) | 23 (100) | 149 (24-858) [0.43] | 16 (69.6) [0.98] | 23 (100) | 3094 (1757-9385) [0.11] | 23 (100) [0.45] | <0.001 | 0.016 |
| mTOR inhibitors (N = 4) | 4 (100) | 11 (1-472) [0.20] | 1 (25) [0.06] | 4 (100) | 72 (37-1839) [0.047] | 2 (50) [0.009] | 0.068 | 0.34 |
AU, arbitrary unit; CDK, cyclin-dependent kinase; IgG, immunoglobulin G; IQR, interquartile range; mTOR, mechanistic target of rapamycin; PARP, poly (ADP-ribose) polymerase; TKIs, tyrosine kinase inhibitors.
Seroconversion response rates at cut-off of 50 AU/ml.
Figure 1.
Cytotoxic therapies and antibody responses after either dose of messenger RNA-BNT162b2 vaccine.
(A) Comparison of distributions and medians of logarithmic IgG titer between treatment subgroups and control at timepoint-2. (B) Comparison of distributions and medians of logarithmic IgG titer between treatment subgroups and control at timepoint-3.
Bars represent median values with 95% confidence interval.
AU, arbitrary units; IgG, immunoglobulin G; RBD S1, receptor binding domain of the S1 subunit of the SARS-CoV-2 spike protein.
Figure 2.
Biological therapies and antibody responses after either dose of mRNA-BNT162b2 vaccine.
(A) Comparison of distributions and medians of logarithmic IgG titer between treatment subgroups and control at timepoint-2. (B) Comparison of distributions and medians of logarithmic IgG titer between treatment subgroups and control at timepoint-3.
Bars represent median values with 95% confidence interval.
AU, arbitrary units; CDK, cyclin-dependent kinase; IgG, immunoglobulin G; mTOR, mechanistic target of rapamycin; PARP, poly (ADP-ribose) polymerase; RBD-S1, receptor binding domain of the S1 subunit of the SARS-CoV-2 spike protein; TKIs, tyrosine kinase inhibitors.
Multivariate analysis of immunogenicity
According to multivariate analysis, treatment with alkylating agents and TKIs significantly reduced variations in IgG titer after the first dose of vaccine, but only treatment with mTOR inhibitors resulted in a similar interaction after both doses. CDK4/6 treatment, however, was independently associated with an incremental variation in IgG titer after the second dose of vaccine. Male sex, older age, ECOG PS2, immunosuppressive corticosteroid dosing, and G-CSF use were all found to be independently associated with a decrease in IgG titer change after the first or second vaccine dose (Table 2). There was a significant relationship between specific treatment subgroups (antimetabolites, alkylating agents, multiple cytotoxic agent regimens, and TKIs) and lack of seroconversion after the first dose of vaccine, but this correlation did not hold at the second dose evaluation. The same analysis confirmed that some clinical features had a negative predictive value for seroconversion response after the first dose, but only advanced line treatment of metastatic disease was associated with a lack of seroconversion after the second dose (Table 3).
Table 2.
Multivariate analysis of anti-RBD S1 logarithmic IgG titer
| Variables | First dose of vaccine |
Second dose of vaccine |
||
|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | |
| Sex | ||||
| Male versus female | −0.69 (−0.17 to 0.03) | 0.18 | −0.07 (−0.14 to −0.002) | 0.045 |
| Age (years) | ||||
| >55 versus ≤55 | −0.14 (−0.26 to −0.02) | 0.017 | −0.06 (−0.14 to 0.02) | 0.45 |
| ECOG PS | ||||
| 0a | — | — | — | — |
| 1 | −0.04 (−0.15 to 0.06) | 0.43 | −0.01 (−0.09 to 0.06) | 0.69 |
| 2 | −0.32 (−0.50 to −0.13) | 0.001 | −0.23 (−0.38 to −0.09) | 0.001 |
| Treatment setting | ||||
| Adjuvant/neoadjuvanta | — | — | — | — |
| Metastatic first line | 0.001 (−0.11 to 0.11) | 0.98 | −0.14 (−0.09 to 0.06) | 0.49 |
| Metastatic second or higher line | 0.005 (−0.13 to 0.14) | 0.94 | −0.34 (−0.13 to 0.06) | 0.11 |
| Corticosteroid therapy | ||||
| yes versus no | −0.14 (−0.29 to −0.007) | 0.04 | −0.11 (−0.21 to −0.02) | 0.016 |
| G-CSF therapy | ||||
| yes versus no | −0.28 (−0.49 to −0.08) | 0.007 | −0.11 (−0.21 to 0.02) | 0.11 |
| Cytotoxic therapy subgroup | ||||
| Controla | — | — | — | — |
| Antimicrotubule agents | −0.02 (−0.22 to 0.18) | 0.85 | 0.01 (−0.13 to 0.15) | 0.85 |
| Topoisomerase inhibitors | −0.07 (−0.32 to 0.18) | 0.58 | −0.15 (−0.34 to 0.03) | 0.11 |
| Antimetabolites | −0.13 (−0.30 to 0.03) | 0.12 | −0.01 (−0.13 to 0.10) | 0.77 |
| Alkylating agents | −0.16 (−0.32 to −0.002) | 0.047 | −0.07 (−0.18 to 0.03) | 0.20 |
| Multiple agents | −0.15 (−0.31 to 0.003) | 0.054 | −0.09 (−0.20 to 0.01) | 0.09 |
| Biological therapy subgroup | ||||
| Controla | — | — | — | — |
| Hormonal therapy | −0.11 (−0.35 to 0.13) | 0.37 | 0.37 (−0.13 to 0.87) | 0.15 |
| Immune checkpoint inhibitors | −0.10 (−0.28 to 0.08) | 0.27 | 0.02 (−0.35 to 0.39) | 0.91 |
| PARP inhibitors | −0.10 (−0.48 to 0.27) | 0.57 | 0.19 (−0.58 to 0.96) | 0.62 |
| TKIs | −0.21 (−0.41 to −0.01) | 0.039 | −0.01 (−0.41 to 0.37) | 0.92 |
| Monoclonal antibodies | −0.005 (−0.19 to 0.18) | 0.96 | 0.20 (−0.17 to 0.57) | 0.28 |
| CDK4/6 inhibitors | 0.04 (−0.17 to 0.26) | 0.67 | 0.47 (0.04 to 0.90) | 0.03 |
| mTOR inhibitors | −0.55 (−1.03 to −0.06) | 0.027 | −0.9 (−1.76 to −0.04) | 0.039 |
AU, arbitrary unit; CDK, cyclin-dependent kinase; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; G-CSF, granulocyte colony-stimulating factor; IgG, immunoglobulin G; mTOR, mechanistic target of rapamycin; PARP, poly (ADP-ribose) polymerase; RBD S1, receptor binding domain of the S1 subunit of the SARS-CoV-2 spike protein; TKIs (tyrosine kinase inhibitors).
Reference category.
Table 3.
Multivariate analysis of seroconversion response at cut-off of 50 AU/ml
| Variables | First dose of vaccine |
Second dose of vaccine |
||
|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | |
| Sex | ||||
| Male versus female | −0.39 (−0.90 to 0.11) | 0.90 | −0.11 (−0.95 to 0.73) | 0.79 |
| Age (years) | ||||
| >55 versus ≤55 | −0.58 (−1.20 to 0.02) | 0.059 | −0.03 (−1.03 to 0.97) | 0.95 |
| ECOG PS | ||||
| 0a | — | — | — | — |
| 1 | −0.31 (−0.85 to 0.22) | 0.24 | 0.18 (−0.78 to 1.15) | 0.70 |
| 2 | −1.62 (−2.65 to −0.60) | 0.002 | −1.32 (−2.68 to 0.02) | 0.054 |
| Treatment setting | ||||
| Adjuvant/neoadjuvanta | — | — | — | — |
| Metastatic first line | 0.04 (−0.55 to 0.63) | 0.89 | −0.47 (−1.56 to 0.60) | 0.38 |
| Metastatic second or higher line | −0.20 (−0.92 to 0.50) | 0.56 | −1.27 (−2.45 to −0.09) | 0.034 |
| Corticosteroid therapy | ||||
| yes versus no | −0.53 (−1.22 to 0.15) | 0.13 | −0.47 (−1.48 to 0.53) | 0.35 |
| G-CSF therapy | ||||
| yes versus no | −1.28 (−2.37 to −0.20) | 0.02 | −1.21 (−2.62 to 0.19) | 0.09 |
| Cytotoxic therapy subgroup | ||||
| Controla | — | — | — | — |
| Antimicrotubule agents | −0.57 (−1.61 to 0.47) | 0.28 | 0.71 (−1.05 to 2.48) | 0.42 |
| Topoisomerase inhibitors | −0.87 (−2.22 to 0.47) | 0.20 | −0.28 (−2.02 to 1.45) | 0.75 |
| Antimetabolites | −1.13 (−1.98 to −0.29) | 0.008 | 0.82 (−0.70 to 2.36) | 0.29 |
| Alkylating agents | −0.80 (−1.60 to −0.002) | 0.049 | 0.46 (−0.88 to 1.81) | 0.50 |
| Multiple agents | −0.99 (−1.79 to −0.20) | 0.014 | 0.27 (−1.00 to 1.55) | 0.67 |
| Biologic therapy subgroup | ||||
| Controla | — | — | — | — |
| Hormonal therapy | −0.32 (−1.56 to 0.91) | 0.60 | 0.30 (−1.97 to 2.58) | 0.79 |
| Immune checkpoint inhibitors | −0.46 (−1.42 to 0.49) | 0.34 | 1.37 (−0.84 to 3.58) | 0.22 |
| PARP inhibitors | −1.22 (−2.99 to 0.54) | 0.17 | 20.53 (NA) | 1.00 |
| TKIs | −1.23 (−2.24 to −0.23) | 0.015 | 0.13 (−1.42 to 1.70) | 0.86 |
| Monoclonal antibodies | −0.39 (−0.73 to 1.53) | 0.49 | 0.46 (−1.26 to 2.18) | 0.60 |
| CDK4/6 inhibitors | −0.17 (−1.33 to 0.97) | 0.76 | 20.80 (NA) | 0.99 |
| mTOR inhibitors | −2.31 (−4.78 to 0.14) | 0.065 | −1.71 (−3.99 to 0.55) | 0.13 |
AU, Arbitrary Unit; CDK, cyclin-dependent kinase; CI, Confidence Interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; G-CSF, granulocyte colony-stimulating factor; mTOR, mechanistic target of rapamycin; NA, not applicable; PARP, poly (ADP-ribose) polymerase; TKIs, tyrosine kinase inhibitors.
Reference category.
Discussion
The Vax-On trial is one of the most extensive studies on the safety and immunogenicity of any COVID-19 vaccine in patients with solid cancer who are receiving active treatment. Our preliminary findings showed that patients on treatment had a significantly lower anti-RBD S1 IgG titer and seroconversion rate after the first dose when compared with those who had been off for at least 28 days. These findings suggested that the timing of cancer treatment, rather than the diagnosis of cancer, could impair immune response to the first dose of vaccine. The seroconversion rate of 52% after the first dose was quite similar to that reported in previous studies.13, 14, 15, 16 The second dose of vaccine resulted in a 15-fold increase in median IgG titer in the control group and an even higher increase in the exposed group. This variation increased the rate of seroconversion in both cohorts by up to 90% or more, overcoming the negative effect of treatment on immune response. The 91% seroconversion rate within the exposed cohort was broadly consistent with the same figure reported in similar studies.14, 15, 16, 17, 18, 19, 20, 21 Although univariate analyses of most previous studies have described cytotoxic chemotherapy13,15,16,18, 19, 20 or its combination with biologic agents17 as having a negative effect on antibody response, our testing did not confirm this interaction. We initially used the same generic classification of active cancer treatment as the other studies to ensure a reliable comparison. As for cytotoxic chemotherapy and targeted therapies, however, this classification is oversimplified because it ignores the different immunosuppressive potentials of each drug class on humoral response.22,23 It is noteworthy that active cancer therapy modulates the immune response to influenza vaccines depending on the type of treatment.24 This secondary analysis was carried out to overcome this generalization by evaluating the immunogenicity of the tozinameran vaccine in relation to a comprehensive classification of anticancer agents. Cytotoxic agents that directly (alkylating) or indirectly (topoisomerase inhibitors and antimetabolites) damage DNA molecules, combination chemotherapy, and TKIs have been linked to significantly lower antibody and seroconversion responses after priming, according to univariate testing. Despite the fact that multivariate analysis confirmed their negative impact on seroconversion, only alkylating agents and TKIs were independently correlated with a lower IgG titer after the first vaccine dose. While such an interaction has been described for TKIs in the treatment of patients with hematologic malignancies,25 it has never been documented for alkylating agents. The second dose resulted in a significant increase in IgG titers in all subgroups, although the seroconversion rate (71%) for patients receiving topoisomerase inhibitors was inadequate. This finding is being reported for the first time, though it may be indirectly consistent with preclinical models that have already shown a negative interaction between topoisomerase-1 inhibition and the immune response caused by SARS-CoV-2 infection.26 The results of our multivariate analysis provide new insights into two aspects. Treatment with mTOR inhibitors was the only one that was independently linked to a reduction in IgG titer after both doses of the vaccine. The very small size of the latter subgroup may reflect a falsely positive outcome. This interaction, although intuitive because of the strong immunosuppressive properties of the drug class, has never been described before and cannot be reliably confirmed. CDK4/6 inhibitors, however, were even associated with an incremental change in IgG titer when compared with the control. The latter finding receives mixed confirmation in subgroup analyses of small samples from similar studies.18,27 Our data would also shed light on the immune response impairment caused by supportive cancer therapies and specific clinical settings, especially after the second dose. While immunosuppressive corticosteroid dosage has been shown to have a negative effect on vaccine immunogenicity,28 a similar negative interaction with G-CSF therapy has not been previously described. Similarly, the reduced immunogenicity of the tozinameran vaccine in cancer patients with unfavorable clinical conditions, such as ECOG PS2, is reported for the first time.
There are several limitations to this subgroup analysis of the Vax-On study that must be acknowledged. First, the sample size was not based on statistical hypothesis testing. Due to health emergency, we adopted an ‘all-comers’ design, which did not allow for adequate subgroup stratification of enrolled patients. As a result, the study may have been prone to selection bias. For the same reasons, we are aware that false-positive results from multivariable statistical comparisons may be amplified and must be regarded as exploratory. Second, because the study design did not require a negative serologic titer or a negative SARS-CoV-2 real-time RT-PCR swab test at baseline, we cannot rule out the possibility that previous viral exposure induced a high-level seroconversion response. Because the study was conducted during a period of compelling social constraints, however, we believe that any unrecognized COVID-19 infections had a negligible impact. Third, a significant proportion of patients (8%) did not receive serologic testing following the second dose. This rate of drop-out is expected in clinical trials involving a large number of cancer patients with advanced disease. Finally, similar to previous studies, the current study assumed anti-RBD S1 IgG titer as a correlate of neutralizing antibodies against SARS-CoV-2, as well as seroconversion as a surrogate for protection against COVID-19 disease. Although suggested by clinical evidence, the first correlation has yet to be established.29 Similarly, the level of anti-RBD S1 antibody titer that provides adequate protection against COVID-19 infection is still debatable, and accurate surrogates in the clinical setting are lacking.30
Conclusion
Collectively, our findings suggest a complex interaction between the pharmacodynamic properties of antineoplastic agents and the immunogenicity of tozinameran vaccine. Drugs interfering with DNA synthesis, multiple-agent chemotherapy, TKIs, and mTOR inhibitors hamper the humoral response. This detrimental effect appears to be more pronounced after the first dose of the vaccine, which is consistent with our previous results. These findings are reported for the first time and are presumed to be more comprehensive than the negative predictive value of generic chemotherapy described in other studies. Because of the lack of reliable comparisons and the minimal size of some subgroups, our results should be considered exploratory and warrant further testing in larger series to be valuable in prioritizing high-risk patients for the third dose of vaccine.
Acknowledgements
This study is dedicated to the patients, their families, and all the nursing, technical, and administrative staff, whose voluntary efforts have made it possible.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.The Lancet Oncology COVID-19: global consequences for oncology. Lancet Oncol. 2020;21:467. doi: 10.1016/S1470-2045(20)30175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillmore N.R., La J., Szalat R.E., et al. Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study. J Natl Cancer Inst. 2020;113:691–698. doi: 10.1093/jnci/djaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robilotti E.V., Babady N.E., Mead P.A., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong X., Qi Y., Huang J., et al. Epidemiological and clinical characteristics of cancer patients with COVID-19: a systematic review and meta-analysis of global data. Cancer Lett. 2021;508:30–46. doi: 10.1016/j.canlet.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelli F., Fabbri A., Onorato A., et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: preliminary results from the prospective observational Vax-On study. Ann Oncol. 2021;20:S0923–S7534. doi: 10.1016/j.annonc.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BC Cancer Agency Cancer Drug Manual Drug Index. Vancouver, British Columbia [updated continuously] 2021. http://www.bccancer.bc.ca/health-professionals/professional-resources/cancer-drug-manual/drug-index/ Available at.
- 11.SARS-CoV-2 immunoassays. https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2/ Available at.
- 12.AdviseDx SARS-CoV-2 IgG II Package insert. Abbott Laboratories. 2021. https://www.fda.gov/media/146371/download/ Available at.
- 13.Palich R., Veyri M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32:1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Noia V., Pimpinelli F., Renna D., et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021;27:6815–6823. doi: 10.1158/1078-0432.CCR-21-2439. [DOI] [PubMed] [Google Scholar]
- 17.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 Messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waidhauser J., Schuh A., Trepel M., Schmalter A.-K., Rank A. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunol Immunother. 2020;69:147–157. doi: 10.1007/s00262-019-02449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allegrezza M.J., Conejo-Garcia J.R. Targeted therapy and immunosuppression in the tumor microenvironment. Trends Cancer. 2017;3:19–27. doi: 10.1016/j.trecan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau B., Loulergue P., Mir O., et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23:450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 25.Maneikis K., Šablauskas K., Ringelevičiūtė U., et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:583–592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho J.S.Y., Mok B.W., Campisi L., et al. TOP1 inhibition therapy protects against SARS-CoV-2-induced lethal inflammation. Cell. 2021;184:2618–2623. doi: 10.1016/j.cell.2021.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grinshpun A., Rottenberg Y., Ben-Dov I.Z., Djian E., Wolf D.G., Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021;6:100283. doi: 10.1016/j.esmoop.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vormehr M., Lehar S., Kranz L.M., et al. Dexamethasone premedication suppresses vaccine-induced immune responses against cancer. Oncoimmunology. 2020;9:1758004. doi: 10.1080/2162402X.2020.1758004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lustig Y., Sapir E., Regev-Yochay G., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P., Li J., Pan H., Wu Y., Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: the evidence we have versus the evidence we need. Signal Transduct Target Ther. 2021;6:1–6. doi: 10.1038/s41392-021-00481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.