Abstract
Streptozotocin (STZ) 60 mg/kg, i.p.-induced diabetes in rat’s results into hyperglycemia, impaired oxidative stress, lipid profile, insulin levels and changes in body weight. Treatment with antihyperglycemics and antioxidants are accounted to produce favorable effect in this paradigm. Fustin, a flavonoid derived from Rhus verniciflua, extract of Rhus verniciflua reported to exhibit anti-hyperglycemic, antioxidant, anti-microbial, anti-arthritic effects, anti-obesity effects, antiplatelet effects and anti-cancer effects. However, no evidence is existing on effect of fustin on STZ-induction diabetes. Thus, we evaluated its effects against diabetes in STZ-induced rodents. Blood glucose, Insulin, lipid peroxidation (MDA), superoxide dismutase (SOD), catalase activity (CAT), glutathione (GSH) and lipid profile levels was assessed. After 30 days diabetes induction rodents showed a severe increased blood sugar level, MDA, high density lipid and decreased cholestrol, triglyceride, GSH, SOD, CAT, respectively.
Oppositely, treatment with fustin (50–100 mg/kg/p.o., two times daily, 30 days) enhanced blood glucose, lipid profile levels Insulin. Meanwhile, reduced MDA and enhanced GSH, SOD, and CAT in diabetic rats. Glibenclamide 5 mg/kg/p.o. also enhanced diabetes-induced complications and decreased oxidative stress. Further histopathology of pancreas confirms the protective effect fustin in STZ-induction diabetes in animals. In conclusion, the study revealed treatments with fustin avoid the changes in body weight, blood glucose, lipid profile and oxidative stress. As a results of these finding may lead to the growth of a choice of medicine for hyperglycemic in the future.
Keyword: Blood glucose, Body weight, Diabetes, Fustin, Glyburide, Lipid profile, Oxidative stress
1. Introduction
Diabetes mellitus (DM) is now the world's largest healthcare issues despite the understanding of the pathogensis of DM, existing medicine only have a temporary antidiabetic result and have unsuccessful to fully avoid the progression of these abnormality (Lotfy et al., 2017). Diabetes is linked to long-term disorders including retinal vascular disease, neuropathy, diabetic kidney disease and blood vessels cell damage. Moreover, diabetes is regarded as a significant risk of cardiac comorbidities, which leads to increased mortality in diabetic patients (Gregg et al., 2014).
Chronic hyperglycemia and defects in lipid panel, such as triglycerides, cholesterol, lower- and higher-density lipid and characterise certain diabetes conditions, leading to a number of secondary complications (Gabir et al., 2000). As a result, some hypoglycemic agents have been used to cure DM; however they have severe adverse outcomes such as hepatic problem, lactic acidosis, and diarrhea (Pari and Saravanan, 2004). Several oral DM medications present, each with a acting on different binding receptor for stimulating insulin (Vasconcelos et al., 2011). Natural plant-based medications, on the other hand, have a variety of biological functions, including antioxidant (Vinayagam and Xu, 2015), antibacterial (Parham et al., 2020), anti-diabetic (Gupta et al., 2017), and anticancer effects (Zhang et al., 2017).
The study reports have established the role of some essential pathways responsible for causing oxidative stress in diabetes, such as polyol, glycation end products (AGEs), and glucose autoxidation (Florez et al., 2010, Turk, 2010, Bandeira et al., 2013, Nowotny et al., 2015, Bell et al., 2013). The available standard medication for diabetes is combined with the use of drug therapy for the rest of one's life, which increases the risk of developing life-threatening extreme adverse events or health complications. The treatment regimen requires the use of medications such as sulfonylureas, a-glucosidase inhibitors, and biguanides, as well as non-pharmacological management such as diet and exercise therapy (Florez et al., 2010, Bell et al., 2013).
Flavonoids, are compounds found in fruits, vegetables, and herbal medicinal plants, have been shown to have anti-diabetic properties in recent studies. (Vinayagam and Xu, 2015). Fustin seems to be a compound derived from Rhus verniciflua Stokes, a traditional herbal medicinal plant from the Anacardiaceae family (Li et al., 2020), is used to treat a variety of illnesses including hyperglycemia (heartwood), helminthiasis, and menstrual problems (Sun et al., 2014). Rhus verniciflua extracts have anti-oxidant (Kim et al., 2002, Kim et al., 1997a), anti-microbial (Kim et al., 1997a, Kim et al., 1997b), anti-mutagenic activity (Park et al., 2004), anti-arthritic effects (Choi et al., 2003), anti-obesity effects (Kim et al., 2003), antiplatelet effects (Jeon et al., 2006) and anti-cancer effects (Jeong et al., 2008, Lee et al., 2009). Similarly, another plant from the Rhus genus (Rhus verniciflua) is aboundent in polyphenolic constituents and has antitumor and anti-inflammatory potentials (Kim et al., 2013). The Verniciflua bark contains an important active constituent known as flavonoid, which has been found to have potent neuroprotective action and may be a potential candidate for cognitive enhancing therapeutic activity (Cho et al., 2013).
However, the extensive antioxidant and anti-diabetic capacity of this bio-constituent in a diabetic animal model has yet to be recorded. Thus, the new protocol addresses fustin anti-diabetic function in an experimental animal paradigm of diabetes.
2. Research design
2.1. Animals
150–200 g adolescent adult Wistar rats were housed and acclimatized for ten days in standard laboratory conditions, which included (n = 6) a module of 12 h light and dark cycle, 22 ± 2 °C temperature, and 50–60% controlled humidity with voluntary approach to rodent chow and tap drinking water. The animal ethics authority for at the institute (RKDFCP/IAEC/2020/33) approved the use of rodents in the experiments and the research was carried out at RKDFCP, India.
2.2. Drugs and chemicals
Fustin and STZ (Streptozotocin) were supplied by the Merk Pvt. ltd. India.
2.3. Design of experiments
2.3.1. Induction of diabetes to experimental animals
We used previously described method by Umathe, et al., 2009 to cause hyperglycemia in rats. (Umathe et al., 2009). In succinct , rats were given STZ (60 mg/kg, i.p.) prepared in 0.1 M cold citrate buffer (pH 4.5) and then given a 5 percent glucose solution instead of water for the next 24 h to prevent death from hypoglycemia. To measure blood glucose levels, blood samples were taken from the tail vein 48 h after STZ or vehicle injection. Only diabetic animals with fasting blood glucose levels greater than 250 mg/dl were used in the study.
2.3.2. The demonstration of experimental protocol
The rats randomized in total five groups such as 1st Group (standard control); 2nd Group (Hyperglycemic control); 3rd Group (fustin dosed 50 mg/kg/p.o.); 4th Group (fustin dosed 100 mg/kg/p.o.) (Moon et al., 2015, Jin et al., 2009); 5th Group(glibenclamide dosed 5 mg/kg/p.o. as standard treatment) for 30 days. Despites of control group which receive saline or vehicle other treatment groups received the dosing of standard or testing scheduling from 1st day to till 30th days. Glucose levels in the blood as well as weight of animals were measured at the start and end of the study.
2.4. Biochemical analysis
2.4.1. Estimation of blood glucose levels
We used a strip-operated blood glucose monitor (Accu chek strips, Roche Diabetes Care) to test glucose levels in blood samples collected by tail prick to establish diabetes mellitus induction. In brief, a droplet of blood is inserted into the glucometer to determine blood glucose levels, and tail veins of the 6 h starved rats were ruptured and a droplet of blood was deposited on the glucometer strip loaded in the glucometer for blood glucose testing. Blood glucose levels were checked periodically during the trial (7, 14, 21 and 30 days after the beginning of treatment).
2.4.2. Evaluation of blood fat levels and liver function test
The serum was isolated from blood samples stored in sterile tubes without an anticoagulant by centrifuging for 10 min at room temperature. The samples were held below −50 °C until total amount of cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) levels and aspartate transaminase, alanine aminotransferase enzymes of hepatic function test- were calculated at the end of experiments as per standard kits.
2.4.3. Estimation of serum insulin levels
Insulin level in the serum was enumerated using immunological assay (ELISA) kits and previously reported methods and protocols recommended by manufactures with slight possible modifications. In line of protocol serum were collected from the all animals groups and subjected for centrifugation at 14000 rpm for 10 min. as per protocols. All the desired number of coated stripes was place into the holder. Pipetting standard (e.g. insulin), control and serum samples into appropriate wells. After adding working insulin enzyme conjugates, samples were subjected to incubation for 60 min at 20-25°c. After completion of incubation 3 times washing with 300 μl wash buffer were performed. After adding 100 μl samples of TMB substrate were subjected to incubation at room temperature for 15 min. Following the final incubation, required quantity of stopping solution were added to all wells and subjected to absorbance reading on monochromatic microplate reader at 450 nM (Wilkin et al., 1985).
2.4.4. Biochemical indicators assessment
The biochemical indicator assessment was done based on previous reported investigations (Aydın et al., 2017). Serum estimation of oxidative stress biomarkers were carried out by previous reported study protocols with slight medication. In the assessment specimens organ (liver) has been taken from each group with 10% w/v homogenized in separate with homogenizer. For the estimation of presence of different protein content specimen were homogenized in 7.4 pH phosphate buffer saline (PBS) of conc. 10 and 50 mM for determination of malondialdehyde (MDA) by Thiobarbituric acid reactive compounds and antioxidant thiol i.e. (GSH) reduced glutathione level, catalase activity and superoxide dismutase (SOD) (CAT). According to the method for estimation of protein presented by Rosebrough et al. The obtained tissue homogenate was subjected to centrifuge at 10000 rpm for period of 15 min. period (Lowry et al., 1951). The pink chromogen generated after reacting with TBARS, which indicates the creation of MDA as a final result of the lipid peroxidation process, was quantified at 532 nM using a spectrophotometric technique. A standard curve was constructed using an MDA standard against which the sample measurements were plotted. (Ohkawa et al., 1979). Similarly, estimation of GSH were produced yellow colored compound on spectrophotometric evaluation at 405 nM by employing commercial available kits and their manufacturer recommendation for estimation (Ohkawa et al., 1979). Determination of CAT enzyme activity based on earlier reported methods by Sinha include colorimetric estimation at 570 nM in presence of hydrogen peroxide and glacial acetic acid (Tietez, 1969, Sinha, 1972). Determination of superoxide dismutase enzyme activity was estimated by employing 96-well plate reader set to 490 nM and available commercial kits and manufacturer's advice for usage to show the quantity of protein necessary to block 6-hydroxydopamine auto-oxidation (Crosti et al., 1987).
2.5. Statistical analysis
Data analysis was carried out using windows based graph pad prism software (5.02). Results of present study were represented as mean standard error of the mean (SEM). To evaluate the significance levels and depict the difference between the variables among each group, one-way analysis of variance (ANOVA) was employed, followed by a post hoc multiple comparison test. Statistical significance was defined as P values less than 0.05.
3. Results
3.1. Mean body weight
3.1.1. Fustin effect on body weight in rats with STZ-induced diabetes
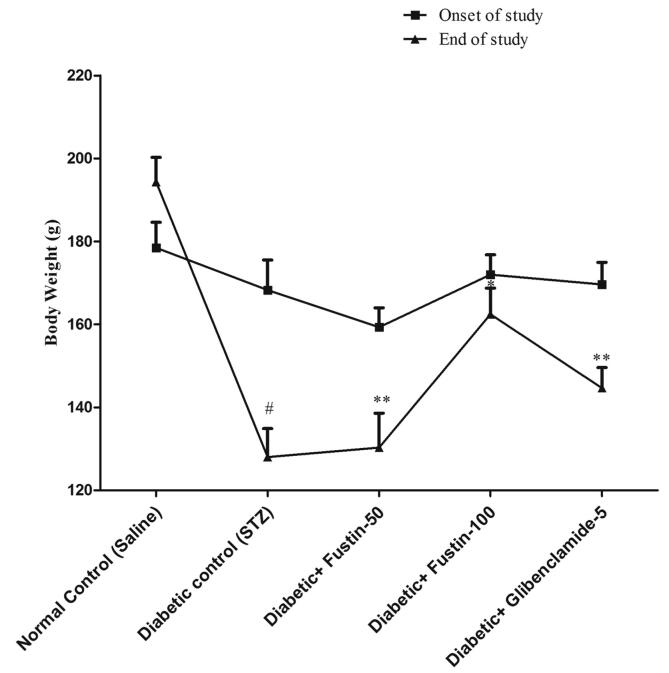
In STZ-induced diabetic rats, the effect of fustin on body weight Fig. 1 depicts a subsequent decrease in mean body weight in the diabetes control group (P 0.01)., while the standard drug glibenclamide treated group greatly reversed the reduction in body weight of the animals in rats, (P < 0.05). The weight loss in animals is slightly restored in the fustin research community with a higher dosage of 100 mg/kg (P < 0.05). The lesser dose of fustin, 50 mg/kg, had minimal effect on body weight, according to a post hoc test.
Fig. 1.
Mean body weight change.
3.2. Blood glucose
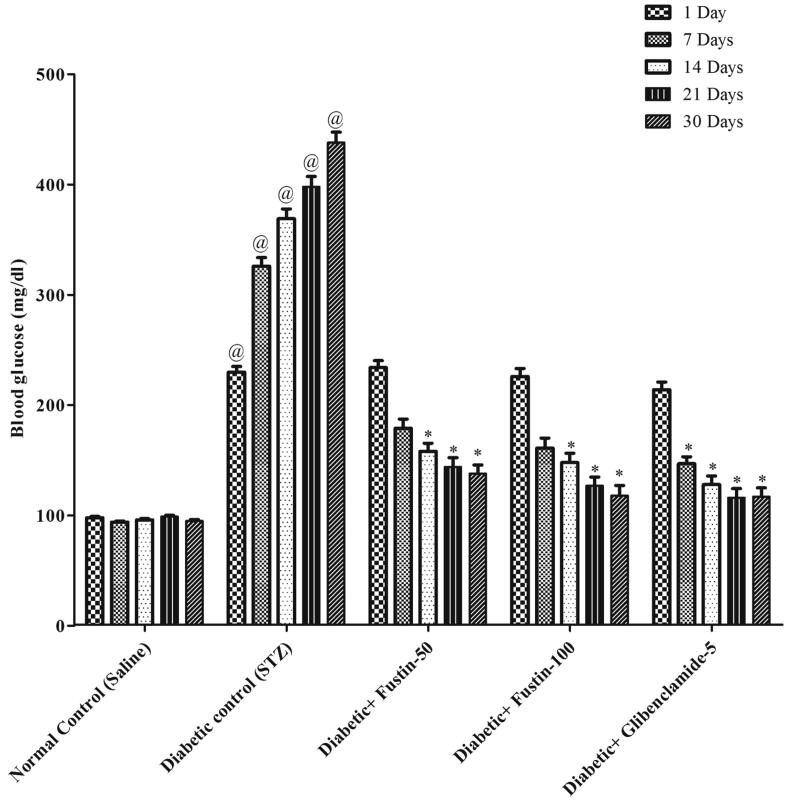
3.2.1. Fustin effect on blood glucose levels in rats with STZ-induced diabetes
The impact of fustin on diabetic rats' blood glucose levels is seen in Fig. 2. From day 14 onwards, the STZ-induced hyperglycemic rats showed a highly important rise (P < 0.001) in blood glucose levels, indicating that diabetes had been successfully inducted in all groups of rats in contrast to normal control rats, which increased further during the experimental phase. By post
Fig. 2.
Effect of fustin on blood glucose level in STZ-induced diabetic rats.
Hoc parametric test study, glibenclamide 5 mg/kg and fustin 100 mg/kg showed a favorable important (P < 0.001) drop in blood glucose after thirty days of therapy, while fustin 50 mg/kg was found to be marginally significant (P < 0.01) as opposed to a diabetic control group (P < 0.001).
3.3. Insulin analysis
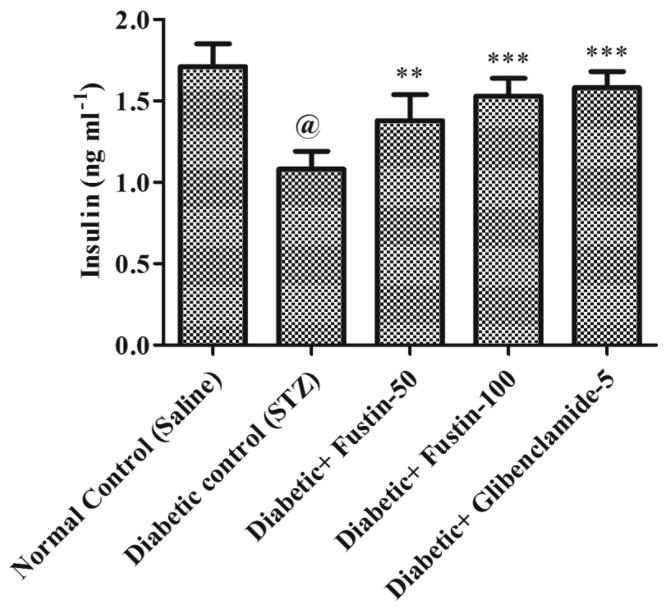
3.3.1. Fustin effect on serum insulin in rats with STZ-induced diabetes
In contrast to normal control groups, the diabetic control group demonstrates a substantial drop (P < 0.001) in serum levels of insulin (Fig. 5). As opposed to diabetic control groups, treatment with the reference medication glibenclamide 5 mg/kg and the research drug fustin 100 mg/kg moderately lowers the elevated levels of serum insulin (P < 0.01). By post hoc parametric test study, a low dose of test drug fustin 50 mg/kg restored elevated insulin levels less dramatically relative to disease control class (diabetes-induced group) (P < 0.05).
Fig. 5.
Effect of fustin on serum insulin in STZ-induced diabetic rats.
3.4. Lipid analysis
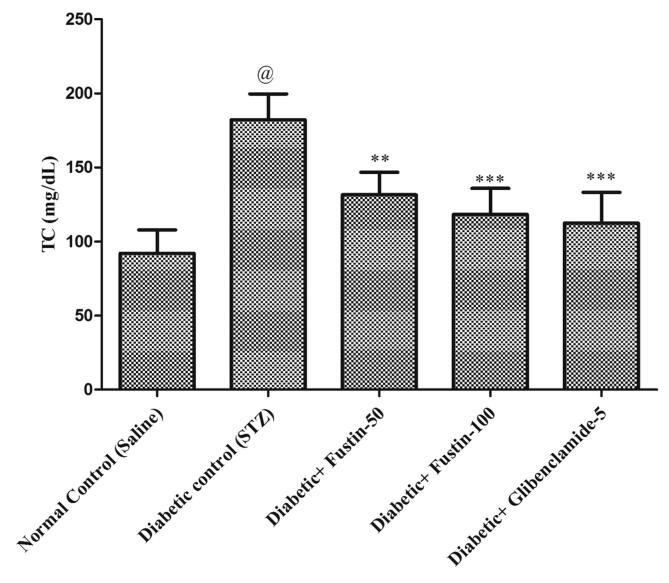
3.4.1. Fustin effect on lipid profile in rats with STZ-induced diabetes
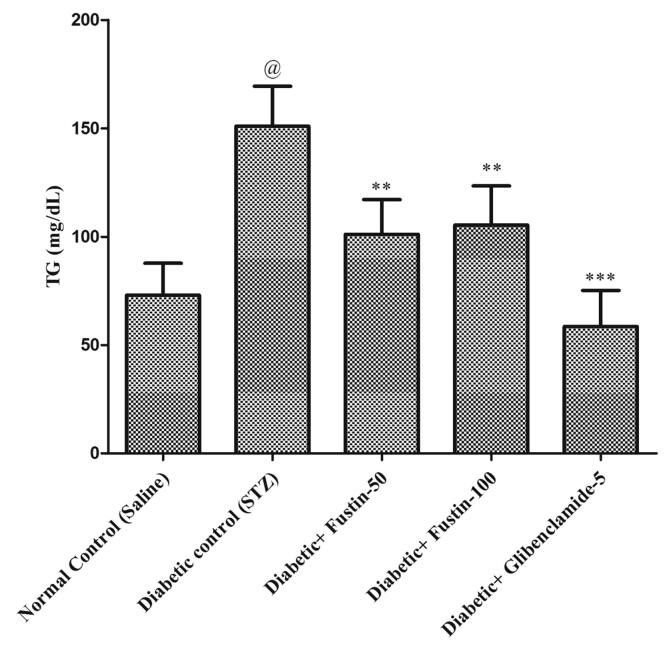
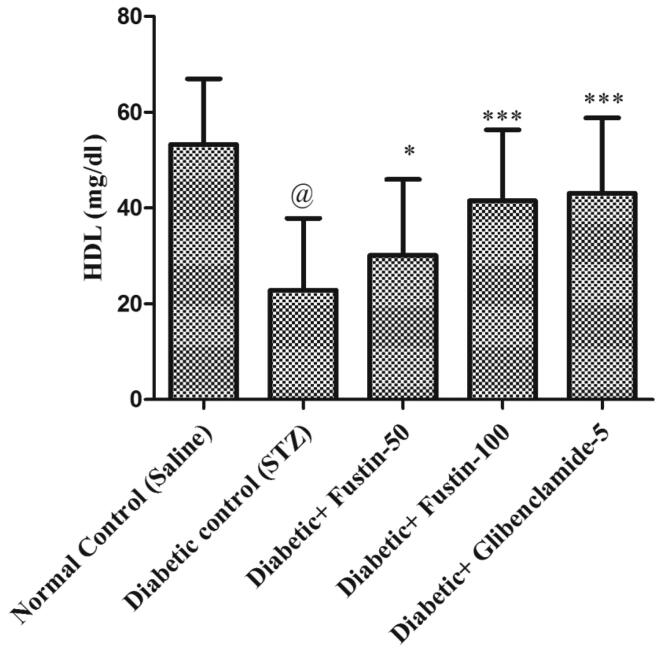
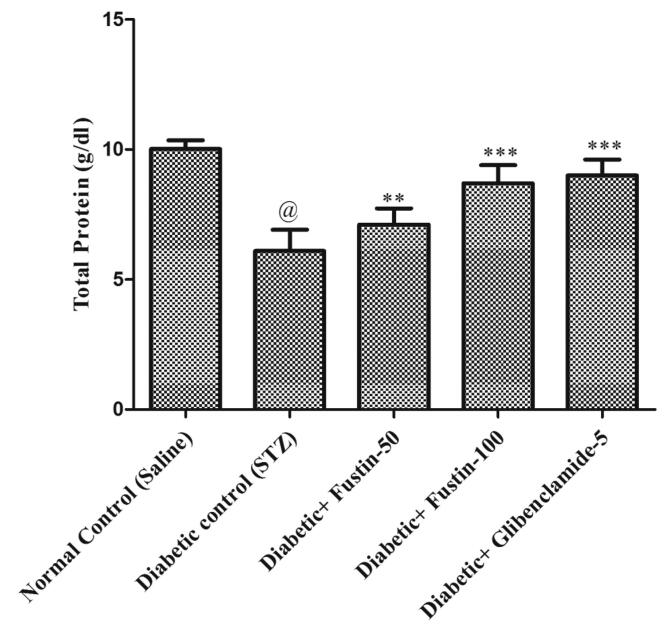
Fig. 3a, Fig. 3b, Fig. 3c, Fig. 3d indicates that diabetic control group serum lipid profiles such as TC, TG and HDL levels exhibit extremely important pathological variation at the conclusion of the experimental procedure. As compared to conventional control groups, the diabetic control group had a considerably higher level of TC and TG in serum (P < 0.001), as well as a considerably lower level of HDL in serum, both of which would be appropriate pathological biomarkers. At the conclusion of the trial regimen, treatment with medication glibenclamide 5 mg/kg and research drug fustin 100 mg/kg substantially attributed the elevated concentrations of serum lipid contour, mostly TC, and TG (P < 0.001). By post hoc parametric test review, the therapy schedule with the lower dose of fustin 50 mg/kg marginally reduces the TC levels (P < 0.01) and less dramatically decreases the levels of TG, and significantly restores the serum levels of HDL (P < 0.05).
Fig. 3a.
Effect of fustin on lipid profile level i.e. TC-Total cholesterol in STZ-induced diabetic rats.
Fig. 3b.
Effect of fustin on lipid profile level i.e.TG- Triglycerides in STZ-induced diabetic rats.
Fig. 3c.
Effect of fustin on lipid profile level i.e. HDL- High density lipoprotein in STZ-induced diabetic rats
Fig. 3d.
Effect of fustin on lipid profile level i.e. TP-total protein in STZ-induced diabetic rats.
3.5. Serum biochemical analysis
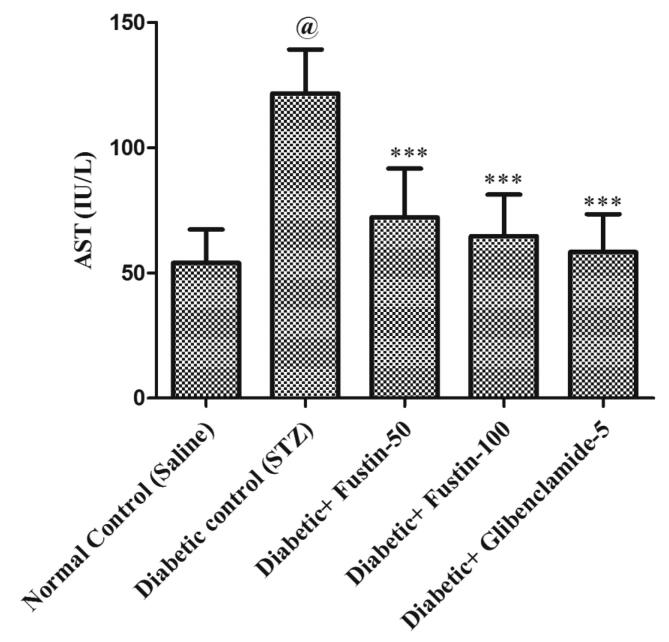
3.5.1. Effect of fustin on serum ALT and AST levels STZ-induced diabetes in rats
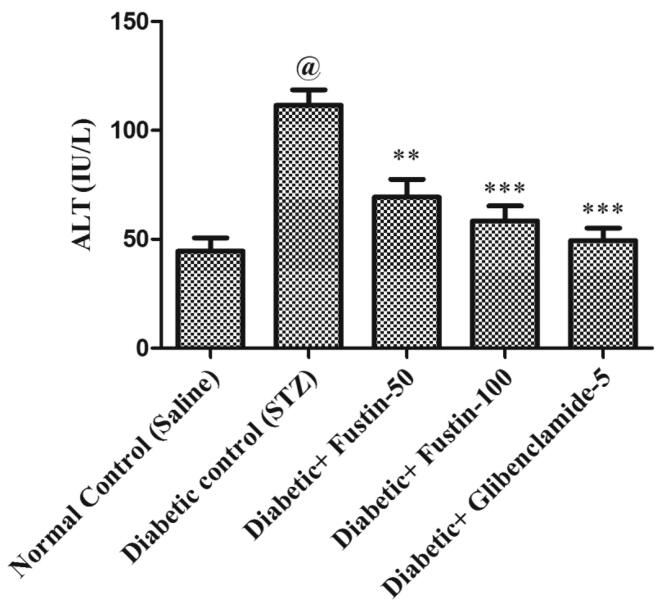
In comparison to standard control groups, the hyperglycaemic control group has a dramatically rise (P < 0.001) in serum amounts of ALT and AST (Fig. 4a, Fig. 4b). Treatment with the standard medication glibenclamide 5 mg/kg and the research drug fustin 100 mg/kg reduced ALT and AST significantly (P < 0.001) in the standard drug management regimen. Via post hoc parametric test study, therapy with a low dose of the test drug fustin 50 mg/kg lowers the increased levels of serum ALT and AST less considerably (P < 0.05).
Fig. 4a.
Effect of fustin on serum AST levels in STZ-induced diabetic rats.
Fig. 4b.
Effect of fustin on serum ALT levels in STZ-induced diabetic rats.
3.6. Oxidative stress biomarkers
3.6.1. Fustin effect on oxidative stress in STZ-induced diabetes rats
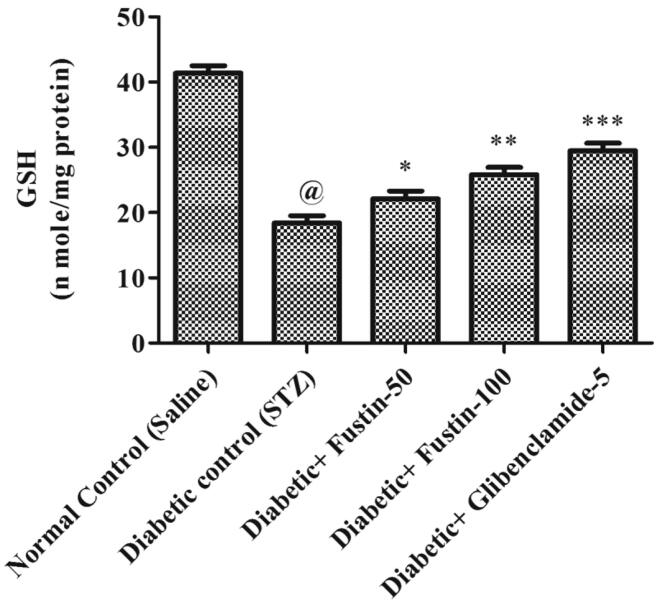
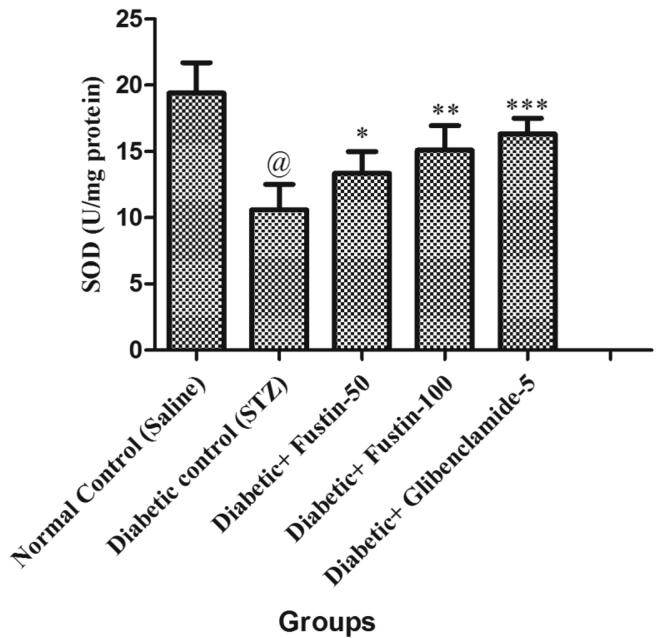
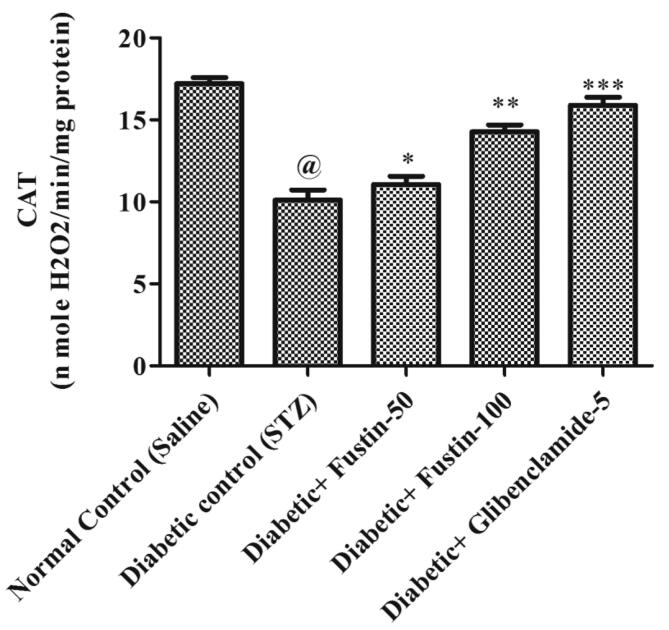
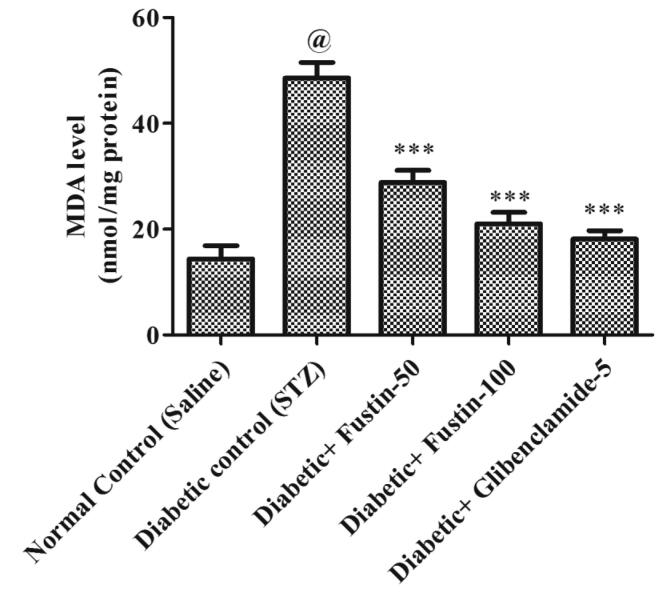
When the study procedure was completed, it was discovered that the diabetic control group had a substantial lower levels of serum GSH, SOD, and CAT (P < 0.001), but notably rise levels of MDA in serum (P < 0.001), when compared to the standard control groups. Treatment with the medication glibenclamide 5 mg/kg and the research drug fustin 100 mg/kg greatly reduces the elevated levels of MDA in the serum (P < 0.001). Furthermore, in comparison to hyperglycaemic test classes, it recovers lower serum levels of GSH, SOD, and CAT. By post hoc parametric test review, a low dose of test drug fustin 50 mg/kg restored the raised levels of MDA in the serum (P < 0.01) less considerably and (P < 0.05) than diabetic control groups, and mildly increased serum levels of GSH, SOD, and CAT (P < 0.01) (Fig. 6, Fig. 7, Fig. 8, Fig. 9).
Fig. 6.
Effect of fustin on hepatic markers of oxidative stress i.e. GSH- reduce glutathione in STZ -induced diabetic rats.
Fig. 7.
Effect of fustin on hepatic markers of oxidative stress i.e. SOD-superoxide dismutase in STZ -induced diabetic rats.
Fig. 8.
Effect of fustin on hepatic markers of oxidative stress i.e. CAT-catalase activity in STZ -induced diabetic rats.
Fig. 9.
Effect of fustin on hepatic markers of oxidative stress i.e. MDA-malondialdehyde in STZ -induced diabetic rats.
3.7. Histopathological studies in pancreas
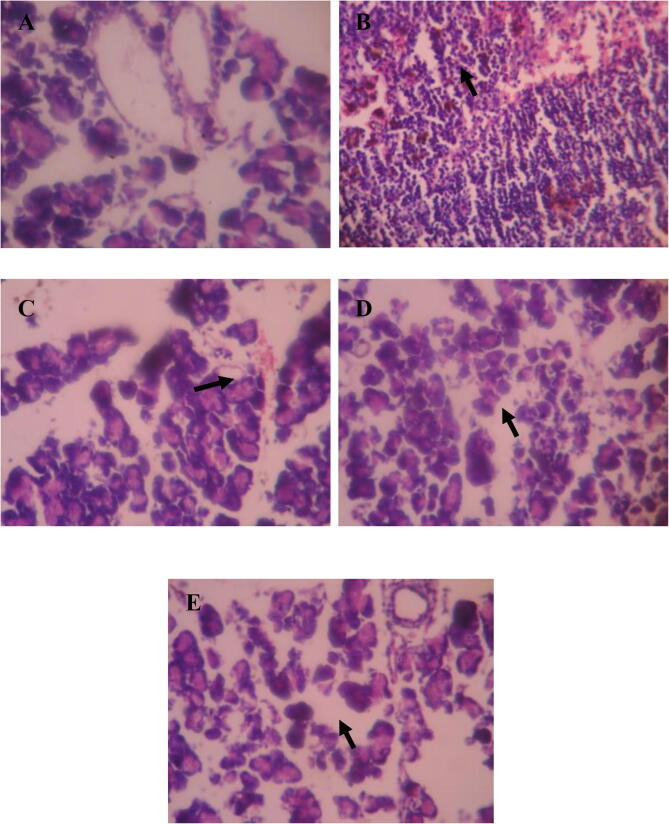
The histological alterations in the pancreatic islets of the five groups were shown in Fig. 10. There were no substantial alterations in pancreatic architecture in normal rats. The islets of STZ-induced diabetic rats displayed severe necrosis and mild atrophy. The diabetic rats show the shrunken islets when compared with the Rats of control group. Following the 30 days of treatment, STZ-induced diabetic rats treated with fustin demonstrated substantial islet expansion and greatly decreased pancreas injuries.
Fig. 10.
Histopathological changes in pancreas A-(normal control); B- (diabetic control); C- (fustin treated 50mg/kg); D-(fustin treated 100mg/kg); E-(glibenclamide 5mg/kg as standard treatment).
4. Discussion
Fustin a flavonoid, was examined to see how it influenced the biochemical activity of diabetic rats. STZ-induced diabetes resulted in specific clinical signs of diabetes, including a loss of body weight, polydipsia, hyperglycemia, and elevated oxidative stress (Guo et al., 2014, Silvares et al., 2016). Chronic fustin therapy dramatically and dose dependently improved body weight loss, polydipsia, hyperglycemia, and elevated oxidative stress. A well-studied experimental diabetes model is streptozotocin-induced diabetes in rats. At the conclusion of the paradigms, STZ generated significant weight reduction, which is consistent with earlier research (Bhutada et al., 2011, Rauter et al., 2010). The present research has showed that after receiving STZ, experimental groups underwent a gradual decrease in body weight. The fundamental clinical correlation for weight loss was thought to be insulin deficiency, which causes protein and fat catabolism (Guo et al., 2014). The treatment of STZ to rats at a dose of 60 mg/kg resulted in an increase in blood glucose levels, which was linked to the loss of pancreatic islets and the death of β-cells.
To establish if fustin has any antihyperglycemic properties, we first looked at blood glucose levels in STZ-induced diabetic rats. The result of our study shows that fustin 50 mg/kg and 100 mg/kg decreases the blood sugar level dose dependently. The results of these finding are in agreement with the previously reported study (Niture et al., 2014). When compared to the control group, glibenclamide 5 mg/kg considerably lowers blood sugar levels.
In the current research, STZ-induced rats had lower insulin levels in their blood than the diabetic control rats, treatment with fustin greatly improved insulin levels in STZ rats. The glibenclamide-treated rats also had insulin levels that were comparable to the fustin treated rats. By regenerating insulin-producing cells, the insulin-mimicking effect of fustin is boosted. The substantial rise in blood insulin levels in STZ-treated rats shows that the hypoglycemic impact of fustin is due to stimulation and potentiation of insulin release from the residual cells of the islets of Langerhans. Surprisingly, our findings agree with those of a prior study (Nain et al., 2012). Several medicinal plant extracts have been shown to activate insulin-producing β-cells in the body. Potentially increasing the release of insulin from β-cells, or regenerating β-cells are the mechanisms through which they exert their anti-hyperglycaemic effects Many other plants may also have anti-hyperglycaemic activity with a stimulatory effect on insulin production as a result of these circumstances (Seedevi et al., 2020). The protective effect of fustin on cells of the islets of Langerhans is also confirmed by histopathology of pancreatic cells The STZ-induced diabetic rats had severe necrosis and mild shrinkage of their islets. Whereas animals given fustin (100 mg/kg) with glibenclamide (5 mg/kg) showed significant islet growth and significantly reduced pancreatic β cell damage. (Rathinam et al., 2014).
Previous studies (Avramoglu et al., 2006, Biddinger et al., 2008) have identified a correlation between dyslipidemia and DM. The widely agreed mechanism is inhibition of lipase activity from pancreas would be fruitful outcome for treatment of metabolic disorders and obesity (Kim et al., 2016). High amounts of triglycerides and total cholesterol, as well as low levels of HDL cholesterol, are consistent with DM in many cases (Hammer and Busik, 2017). TC and TG levels increase in the bloodstream, whereas HDL cholesterol levels fall, leading to secondary complications. The levels of TC, TG and HDL are significant in assessing lipid metabolism in diabetes (Yan et al., 2015). The lipid profile of diabetic rats improved in this study, with higher levels of TC, TG, and lower levels of HDL. In this study, fustin administration reduced TC and TG levels while increasing HDL levels, implying that fustin can boost lipid metabolism to some degree by alleviating dyslipidemia which is a complication of diabetes.
These findings may be linked to the fustin potential defensive effect on pancreatic -cells. Furthermore, inadequate activity of cholesterol synthesising enzymes or a low degree of lipolysis under insulin control might explain these observations. (Belce et al., 2000). As a result, glycol and lipid metabolism can help to boost -cell renovation and insulin secretion.
Diabetes risks include kidney, retinal, cardiac, and liver dysfunctions caused by chronic hyperglycemia. Elevated AST and ALT are a typical symptom of liver disease and are more common in diabetics than in the general population (Rauter et al., 2010). The findings revealed elevated blood glucose levels after diabetes induction and a rise in the levels of liver enzymes (ALT and AST) in diabetic rat’s serum. The substantial rise in ALT and AST levels in diabetic control rats indicated that diabetes mellitus could cause hepatic damage, possibly due to the increase in protein concentrations associated with glucogenesis and urea production found in the diabetic state (Makena et al., 2018). When compared to rats given 50 mg/kg fustin, rats given 100 mg/kg fustin reported a slight rise in ALT and AST levels, suggesting that fustin at 100 mg/kg could have therapeutic effects in the hepatocytes by lowering ALT and AST levels.
Oxidative stress can actively damage various macromolecules in diabetes, including lipids, proteins, and nucleic acids. According to some studies, high blood glucose levels cause an increase in reactive species production as a result of oxidative stress, which leads to a decrease in enzymatic antioxidant levels like SOD and CAT, which leads to the production of free radical scavenging activity, which is then measured for lipid peroxidation. (Strugała et al., 2019). GSH, a non-enzymatic antioxidant, prevents cells against oxidative destruction. This antioxidant is mainly found in the liver and is active in the mechanism of liver detoxification. An experimental model for diabetes showed that serum elevated levels of lipid peroxides (MDA). Furthermore, the study hypothesised that diabetic rats had slightly lower non-enzymatic activity of SOD, GSH, and CAT in liver tissue than usual control rats.
In the current research, it was discovered that the diabetes-controlled community had a higher level of MDA and lower activity of non-enzymatic antioxidants GSH, SOD, and CAT, resulting in substantial oxidative stress in the rat, which would be clinically verified via biochemical review. When the fustin-treated group was exposed to biochemical examination, substantial decreases in lipid peroxide levels were observed, while significant increases in GSH, SOD, and CAT activity.
Furthermore, higher blood glucose levels and reactive oxygen species degradation are connected to increased MDA activation (ROS). Through non-enzymatic glycation of proteins and auto-oxidation, glucose can enhance the production of reactive oxygen species. (Abirami and Kowsalya, 2013). In hyperglycemia, oxidative breakdown of fructosamines can cause oxidative stress. (Greene et al., 1999). In STZ-induced diabetic rats, we found a significant rise in MDA levels and a reduction in SOD, CAT, and GSH levels. Treatment of rats with higher-lower doses of fustin and glibenclamide, on the other hand, lowered MDA levels, returning SOD, CAT, and GSH levels to near-normal levels.. Moreover, fustin treated group showed significant reduction in MDA levels (p < 0.001)
Induction of diabetes with the administration of STZ resulted in weight loss, elevated blood glucose levels, altered liver profile, dyslipidemia, and irregular serum levels of hormones such as insulin all were confirmed through biochemical finding. The ability of fustin to exert strong antioxidant effects by controlling stress biomarkers and producing a reverse effect on low serum insulin levels found in diabetic rat models are possibly involved in the underlying molecular mechanisms of action. If preclinical evidence is validated for clinical trials, fustin will be a pivotal candidate for treating metabolic syndrome in the future.
5. Conclusion
The findings suggest that fustin favourable effect on STZ-induced diabetes may be attributable to its antidiabetic and antioxidant capabilities, which might lead to the development of cost-effective phytochemical options for diabetes management.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program to support publication in the top journal (Grant no. 42-FTTJ-80)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program (Grant no. 42-FTTJ-80) to published in the top journal.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sadaf Jamal Gilani, Email: SJGlani@pnu.edu.sa.
May Nasser Bin-Jumah, Email: mnbinjumah@pnu.edu.sa.
Fahad A. Al-Abbasi, Email: fabbasi@kau.edu.sa.
Muhammad Shahid Nadeem, Email: mhalim@kau.edu.sa.
Muhammad Afzal, Email: afzalgufran@ju.edu.sa.
Imran Kazmi, Email: kazmiimran2005@gmail.com.
References
- Abirami R., Kowsalya S. Antidiabetic activity of Ulva fasciata and its impact on carbohydrate metabol-ism enzymes in alloxan induced diabetic rats. Int. J. Res. Phytochem. Pharmacol. 2013;3:136–141. [Google Scholar]
- Avramoglu R.K., Basciano H., Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin. Chim. Acta. 2006;368:1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Aydın A.F., Bingül İ., Küçükgergin C., Doğan-Ekici I., Doğru Abbasoğlu S., Uysal M. Carnosine decreased oxidation and glycation products in serum and liver of high-fat diet and low-dose streptozotocin-induced diabetic rats. Int. J. Experimental Pathol. 2017;98:278–288. doi: 10.1111/iep.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira D.M., Da Fonseca L.J.S., Guedes D.S., Rabelo L.A., Goulart M.O., Vasconcelos S.M.L. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int. J. Mol. Sci. 2013;14:3265–3284. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belce A., Uslu E., Kucur M., Umut M., Ipbüker A., Seymen H.O. Evaluation of salivary sialic acid level and Cu-Zn superoxide dismutase activity in type 1 diabetes mellitus. Tohoku J. Experimental Med. 2000;192:219–225. doi: 10.1620/tjem.192.219. [DOI] [PubMed] [Google Scholar]
- Bell D.S., Patil H.R., O'keefe, J. H. Divergent effects of various diabetes drugs on cardiovascular prognosis. Rev. Cardiovascular Med. 2013;14:107–122. doi: 10.3909/ricm0671. [DOI] [PubMed] [Google Scholar]
- Bhutada P., Mundhada Y., Bansod K., Tawari S., Patil S., Dixit P., Umathe S., Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011;220:30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Biddinger S.B., Hernandez-Ono A., Rask-Madsen C., Haas J.T., Alemán J.O., Suzuki R., Scapa E.F., Agarwal C., Carey M.C., Stephanopoulos G. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N., Lee K.Y., Huh J., Choi J.H., Yang H., Jeong E.J., Kim H.P., Sung S.H. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol. 2013;58:355–361. doi: 10.1016/j.fct.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Choi J., Yoon B.-J., Han Y.N., Lee K.T., Ha J., Jung H.J., Park H.J. Antirheumatoid arthritis effect of Rhus verniciflua and of the active component, sulfuretin. Planta Med. 2003;69:899–904. doi: 10.1055/s-2003-45097. [DOI] [PubMed] [Google Scholar]
- Crosti, N., Servidei, T., Bajer, J.,Serra, A. 1987. Modification of the 6-hydroxydopamine technique for the correct determination of superoxide dismutase. [PubMed]
- Florez H., Luo J., Castillo-Florez S., Mitsi G., Hanna J., Tamariz L., Palacio A., Nagendran S., Hagan M. Impact of metformin-induced gastrointestinal symptoms on quality of life and adherence in patients with type 2 diabetes. Postgrad. Med. 2010;122:112–120. doi: 10.3810/pgm.2010.03.2128. [DOI] [PubMed] [Google Scholar]
- Gabir M.M., Hanson R.L., Dabelea D., Imperatore G., Roumain J., Bennett P.H., Knowler W.D. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23(8):1113–1118. doi: 10.2337/diacare.23.8.1113. [DOI] [PubMed] [Google Scholar]
- Greene D.A., Stevens M.J., Obrosova I., Feldman E.L. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur. J. Pharmacol. 1999;375:217–223. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- Gregg E.W., Li Y., Wang J., Rios Burrows N., Ali M.K., Rolka D., Williams D.E., Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- Guo C., Zhang C., Li L., Wang Z., Xiao W., Yang Z. Hypoglycemic and hypolipidemic effects of oxymatrine in high-fat diet and streptozotocin-induced diabetic rats. Phytomedicine. 2014;21:807–814. doi: 10.1016/j.phymed.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Gupta R.C., Chang D., Nammi S., Bensoussan A., Bilinski K., Roufogalis B.D. Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndrome. 2017;9:1–12. doi: 10.1186/s13098-017-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer S.S., Busik J.V. The role of dyslipidemia in diabetic retinopathy. Vision Res. 2017;139:228–236. doi: 10.1016/j.visres.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon W.K., Lee J.H., Kim H.K., Lee A.Y., Lee S.O., Kim Y.S., Ryu S.Y., Kim S.Y., Lee Y.J., Ko B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006;106:62–69. doi: 10.1016/j.jep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Park J.W., Yoon S.W., Choi W.C. Carcinostatic effect of allergen removed Rhus verniciflua Stokes based traditional Korean medicine on a patient with lung adenocarcinoma; single case report. Oriental Pharm. Experimental Med. 2008;7:573–578. [Google Scholar]
- Jin C.H., Shin E.J., Park J.B., Jang C.G., Li Z., Kim M.S., Koo K.H., Yoon H.J., Park S.J., Choi W.C., Yamada K., Nabeshima T., Kim H.C. Fustin flavonoid attenuates beta-amyloid (1–42)-induced learning impairment. J. Neurosci. Res. 2009;87:3658–3670. doi: 10.1002/jnr.22159. [DOI] [PubMed] [Google Scholar]
- Kim G.-N., Shin M.-R., Shin S.H., Lee A.R., Lee J.Y., Seo B.-I., Kim M.Y., Kim T.H., Noh J.S., Rhee M.H. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peel. BioMed Res. Int. 2016. 2016 doi: 10.1155/2016/1723042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee H.W., Ko B.S., Kim H.K., Jeon W.K. Effects of Rhus verniciflua Stokes (RVS) extract on diet-induced obesity in C57BL/6 mouse. Korean J. Pharmacognosy. 2003;34:339–343. [Google Scholar]
- Kim K.H., Moon E., Choi S.U., Kim S.Y., Lee K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry. 2013;92:113–121. doi: 10.1016/j.phytochem.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Choi W.C., Barshinikov A., Kobayashi A. Anticancer and antioxidant activity of allergen-removed extract in Rhus verniciflua Stokes. Korean J. Medicinal Crop Sci. 2002;10:288–293. [Google Scholar]
- Kim M.J., Choi Y.H., Kim W.G., Kwak S.S. Antioxidative activity of urushiol derivatives from the sap of lacquer tree (Rhus vernicifera Stokes) Korean J. Plant Resour. 1997;10:227–230. [Google Scholar]
- Kim M.J., Kim C.J., Kwak S.S. Antifungal activity of urushiol components in the sap of Korean lacquer tree (Rhus vernicifera Stokes) Korean J. Plant Resour. 1997;10:231–234. [Google Scholar]
- Lee J.H., Lee H.J., Lee H.J., Choi W.C., Yoon S.W., Ko S.G., Ahn K.S., Choi S.H., Ahn K.S., Lieske J.C., Kim S.H. Rhus verniciflua Stokes prevents cisplatin-induced cytotoxicity and reactive oxygen species production in MDCK-I renal cells and intact mice. Phytomedicine. 2009;16(2-3):188–197. doi: 10.1016/j.phymed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Li M.C., Xie C.J., Gao J.G., Meng C.W., He Y.J., Liu J.Y., Xu Y.N. Chemical constituents from the heartwood of Toxicodendron vernicifluum (Stokes) FA Barkley. Biochem. Syst. Ecol. 2020;90 [Google Scholar]
- Lotfy M., Adeghate J., Kalasz H., Singh J., Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr. Diabetes Rev. 2017;13:3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Makena W., Hamman W.O., Buraimoh A.A., Dibal N.I., Obaje S.G. Therapeutic effects of balanitoside in streptozotocin-induced diabetic rats. Journal of Taibah University Medical Sciences. 2018;13:402–406. doi: 10.1016/j.jtumed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.E., Shin J.H., Kwon O., Kim J.Y. A Standardized Extract of Rhus verniciflua Stokes Protects Wistar Rats Against Lipopolysaccharide-Induced Acute Inflammation. J. Med. Food. 2015;18:1223–1230. doi: 10.1089/jmf.2014.3411. [DOI] [PubMed] [Google Scholar]
- Nain P., Saini V., Sharma S., Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012;142(1):65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Niture N.T., Ansari A.A., Naik S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J Exp Biol. 2014;52:720–727. [PubMed] [Google Scholar]
- Nowotny K., Jung T., Höhn A., Weber D., Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Parham S., Kharazi A.Z., Bakhsheshi-Rad H.R., Nur H., Ismail A.F., Sharif S., Ramakrishna S., Berto F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants. 2020;9:1309. doi: 10.3390/antiox9121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari L., Saravanan R. Antidiabetic effect of diasulin, a herbal drug, on blood glucose, plasma insulin and hepatic enzymes of glucose metabolism in hyperglycaemic rats. Diabetes Obes. Metab. 2004;6:286–292. doi: 10.1111/j.1462-8902.2004.0349.x. [DOI] [PubMed] [Google Scholar]
- Park K.Y., Jung G.O., Lee K.T., Choi J., Choi M.Y., Kim G.T., Jung H.J., Park H.J. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004;90:73–79. doi: 10.1016/j.jep.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Rathinam A., Pari L., Chandramohan R., Sheikh B.A. Histopathological findings of the pancreas, liver, and carbohydrate metabolizing enzymes in STZ-induced diabetic rats improved by administration of myrtenal. J. Physiol. Biochem. 2014;70(4):935–946. doi: 10.1007/s13105-014-0362-z. [DOI] [PubMed] [Google Scholar]
- Rauter A.P., Martins A., Borges C., Mota-Filipe H., Pinto R., Sepodes B., Justino J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin–induced diabetic rats. Phytother. Res. 2010;24:S133–S138. doi: 10.1002/ptr.3017. [DOI] [PubMed] [Google Scholar]
- Seedevi P., Ganesan A.R., Moovendhan M., Mohan K., Sivasankar P., Loganathan S., Vairamani S., Shanmugam A. Anti-diabetic activity of crude polysaccharide and rhamnose-enriched polysaccharide from G. lithophila on Streptozotocin (STZ)-induced in Wistar rats. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-57486-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvares R.R., Pereira E.N.G.D.S., Flores E.E.I., Estato V., Reis P.A., Silva I.J.D., Machado M.P., Neto H.C.D.C.F., Tibiriça E., Daliry A. Combined therapy with metformin and insulin attenuates systemic and hepatic alterations in a model of high-fat diet-/streptozotocin-induced diabetes. Int. J. Exp. Pathol. 2016;97:266–277. doi: 10.1111/iep.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Strugała P., Dzydzan O., Brodyak I., Kucharska A.Z., Kuropka P., Liuta M., Kaleta-Kuratewicz K., Przewodowska A., Michałowska D., Gabrielska J. Antidiabetic and antioxidative potential of the blue Congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules. 2019;24:3126. doi: 10.3390/molecules24173126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Choi Y.H., Na C.S., Lee D., Yoo H.H., Hong C.Y., Ahn B.Y., Dong M.S. Estrogenic activity of a Rhus verniciflua extract and its major components. J. Funct. Foods. 2014;11:250–260. [Google Scholar]
- Tietez, F. 1969. Enzymic method for quantitative determination of nanogram amounts of total and oxidized. [DOI] [PubMed]
- Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol. Res. 2010;59 doi: 10.33549/physiolres.931585. [DOI] [PubMed] [Google Scholar]
- Umathe S.N., Kochar N.I., Jain N.S., Dixit P.V. Gastrointestinal dysfunction in diabetic rats relates with a decline in tissue L-arginine content and consequent low levels of nitric oxide. Nitric Oxide. 2009;20(2):129–133. doi: 10.1016/j.niox.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Vasconcelos C.F.B., Maranhão H.M.L., Batista T.M., Carneiro E.M., Ferreira F., Costa J., Soares L.A.L., Sá M.D.C., Souza T.P., Wanderley A.G. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea Martius bark extract on streptozotocin-induced diabetes in Wistar rats. J. Ethnopharmacol. 2011;137(3):1533–1541. doi: 10.1016/j.jep.2011.08.059. [DOI] [PubMed] [Google Scholar]
- Vinayagam R., Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutrit. Metab. 2015;12:1–20. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin T., Nicholson S., Casey C. A micro enzyme-linked immunosorbent assay for insulin antibodies in serum. J. Immunol. Methods. 1985;76:185–194. doi: 10.1016/0022-1759(85)90490-9. [DOI] [PubMed] [Google Scholar]
- Yan Z., Fan R., Yin S., Zhao X., Liu J., Li L., Zhang W., Ge L. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015;80:573–580. doi: 10.1016/j.ijbiomac.2015.05.054. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang Y., He C. Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chinese Med. 2017;12:1–15. doi: 10.1186/s13020-017-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]