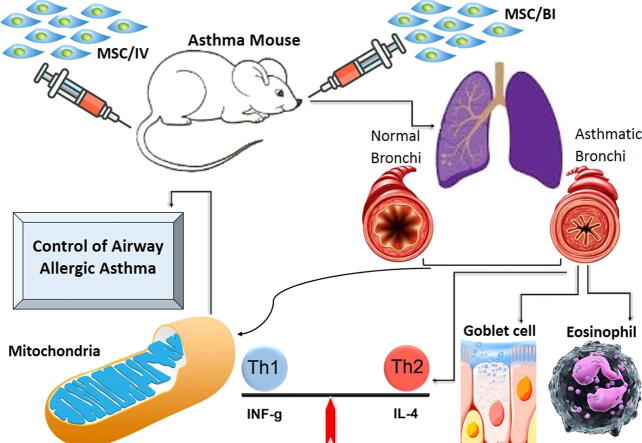
Graphical abstract
Keywords: MSC, Inflammation, Immune system, Allergy
Abbreviations: AHR, Airway hyperresponsiveness; ATP, Adenosine triphosphate; BALF, Bronchoalveolar lavage fluid; BM, Bone marrow; CCL, Chemokine (C-C motif) ligand; CD, Cluster of differentiation; COX, Cyclooxygenase; Cys-LT, Cysteinyl Leukotriene; Cytb, Cytochrome b; ELISA, Enzyme-linked immunosorbent assay; Drp1, Mitochondrial fission depends on the cytosolic GTPase dynamin-related protein 1; FIS1, Mitochondrial fission 1 protein; H&E, Haemotoxylin and eosin; HGF, Hepatocyte growth factor; HLA, Human leukocyte antigen; HO, Heme oxygenase; IDO, Indoleamine 2,3-dioxygenase; IFN, Interferon; Ig, Immunoglobulin; IL, Interleukin; IT, Intratrachea administration; IP, Intraperitoneal injection; iPSC, induced pluripotent stem cells; LT, Leukotriene; MFN, Mitofusin; MIP, macrophage inflammatory protein; MMP, Matrix metalloproteinase; MSC, mesenchymal stem cell; MSC/BI, mesenchymal stem cell bronchial administration; MSC/IV, mesenchymal stem cell intravenous injection; ND1, NADH-ubiquinone oxidoreductase chain 1; NO, Nitric oxide; Nrf, Nuclear erythroid 2 p45-related factor; OPA1, Mitochondrial dynamin like GTPase; OVA, Ovalbumin; PAS, Periodic-acid-Schiff; PBS, Phosphate-buffered saline; PGC1a, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PGE2, Prostaglandin E2; ROS, Reactive oxygen species; TFAM, Transcription factor A mitochondrial; TGF, Transforming growth factor; Th, T helper; TNF, Tumor necrosis factor
Abstract
Asthma is a complicated lung disease, which has increased morbidity and mortality rates in worldwide. There is an overlap between asthma pathophysiology and mitochondrial dysfunction and MSCs may have regulatory effect on mitochondrial dysfunction and treats asthma. Therefore, immune-modulatory effect of MSCs and mitochondrial signaling pathways in asthma was studied.
After culturing of MSCs and producing asthma animal model, the mice were treated with MSCs via IV via IT. BALf's eosinophil Counting, The levels of IL-4, −5, −13, −25, –33, INF-γ, Cys-LT, LTB4, LTC4, mitochondria genes expression of COX-1, COX-2, ND1, Nrf2, Cytb were measured and lung histopathological study were done.
BALf's eosinophils, the levels of IL-4, −5, −13, −25, –33, LTB4, LTC4, Cys-LT, the mitochondria genes expression (COX-1, COX-2, Cytb and ND-1), perivascular and peribronchial inflammation, mucus hyper-production and hyperplasia of the goblet cell in pathological study were significantly decreased in MSCs-treated asthma mice and reverse trend was found about Nrf-2 gene expression, IFN-γ level and ratio of the INF-γ/IL-4.
MSC therapy can control inflammation, immune-inflammatory factors in asthma and mitochondrial related genes, and prevent asthma immune-pathology.
1. Introduction
Asthma is a complicated bronchial disease, which characterized by AHR, eosiniphilic inflammation, mucus hyper-production and airway remodeling. The morbidity and mortality rates of asthma have increased worldwide, and understanding and identification of the molecular mechanisms underlying immune-inflammatory progression of the asthma is necessary to develop new anti-asthma drugs (Athari et al., 2017, Masoume Athari et al., 2018, Mehrabi Nasaba et al., 2020).
There is a considerable overlap between asthma pathophysiology and mitochondrial dysfunction in the aspects of ROS production, oxidative stress, ATP synthase, apoptosis, and calcium homeostasis. Mitochondria are powerhouse organelle in the cells and severely involved into the cell signaling pathways. Mitochondrial regulate cell growth, differentiation, death, autophagy, apoptosis, hypoxic stress responses especially in the signaling of the innate and acquired immune system's cells and therefore, have relation with the various diseases pathophysiology and one of these diseases is allergic asthma. The lung is an oxygen-rich environment and mitochondria are O2 sensor, and generating ROS and also participating in pro-inflammatory signaling pathways. Mitochondrial defects play an important role in the pro-remodeling mechanisms of lung fibrosis and apoptosis of the airway cells. (Lin et al., 2009, Cho et al., 2012).
The MSCs regulate cell function and may have regulatory effect on mitochondrial dysfunction and can transfer and increase healthy mitochondria to the inflamed tissue that treats pathology aspects and structural remodeling of the lung in asthma. MSCs have attracted much attention because of their immune-regulation capacity that aroused to the treatment of asthma. Furthermore, clinical trials have indicated that MSCs have low immunogenicity and can be safe in clinical application. MSCs modulate T-regs proliferation and function by secreting molecules, such as TGF-β1, IL-10, HGF, PGE2, HO-1, IDO, NO, and HLA-G (Ogulur et al., 2014, Yan et al., 2014, Jian-guo et al., 2013). Therefore, study the relationship between immune-modulatory effect of MSCs and mitochondrial signaling pathways in asthma is necessary that the effect of MSCs on the mitochondria signaling pathways in asthma was studied.
2. Material and methods
2.1. BM-MSCs culture
For isolation BM-MSCs, in mice, four claws were dissected at the ankle and carpal joints, and then incisions were made around the connection between hind-limbs and trunk, forelimbs, and trunk. Tibias and femurs are dissected by cutting at the joints and scrubbed to remove the residual tissues and transferred to complete a-MEM medium (10 ml) on ice. After flushing bone marrow out of the cavity, that was cultured in a 5% CO2 incubator (at 37 °C) for 5 days. The initial spindle-shaped cells were appeared on day 3. The cells were re-suspended in a cell culture flask. Passaging was performed every 4e6 days. BM-MSCs for CD44 and CD90, were positive, and for CD45 and CD31 were negative (Ogulur et al., 2014, Huang et al., 2015).
2.2. Animal treatment schedule
40 BALB/c mice (male; 6–8 week-old) were kept 1 week under standard conditions. The mice were divided into 4 groups (n = 10) that include: control group, which was sensitized and challenged with PBS; the three remained groups were sensitized and challenged with OVA to produce asthma model and that received no treatment, and were treated with MSCs via bronchial (MSC/BI) and MSCs via intravenous (MSC/IV). To produce an allergic asthma mouse model, the animal were sensitized by 20 μg OVA plus 50 μL alum on days 1 and 14 (via IP injection) and then were challenged by OVA (1% solution) using a nebulizer (via IT; 30 min/day) on days 24, 26, 28, and 30. One of the asthma groups received MSCs by BI administration (2.5 × 105 cells) on day 25, and the second asthma group received MSCs by IV administration (2.5 × 105 cells) on day 25. In day 31, the blood and BALf were taken after anesthetization of the mice. For BALf sample collection, anesthetized mice were tracheastomized and the lung was lavaged by PBS.Then, the mice were euthanized by CO2 and lung tissue samples were taken.
2.3. Balf's cell Counting
After anesthesia, BALf was collected via intubation, then centrifuged and the supernatant collected for biofactors levels analysis and the cells were fixed to the slide and stained (with Giemsa) to determine the eosinophil percentage.
2.4. Cytokines
The levels of INF-γ, IL-4, −5, −13, −25, and –33 were assayed in BALf by specific ELISA kits according to the manufacturer’s instructions.
2.5. Eicosanoid levels
Cys-LT, LTB4 and LTC4 were measured in BALf using specific ELISA kits.
2.6. Mitochondria isolation and genes expression
The lung tissue was placed in ice-cold homogenization buffer containing 70 mM sucrose (pH 7.4), 10 mM HEPES, 200 mM mannitol, 2% fatty acid-free BSA, 1 mM EGTA, and 50 μL/g tissue protease inhibitor cocktail Set III, and then minced over ice. After homogenizing lung tissue, that was centrifuged at 2,000 × g for 15 min at 4 °C. The supernatant was centrifuged (at 17,800 × g) for 15 min at 4 °C and then re-suspended in ice-cold homogenization solution (5 ml) and centrifuged at (17,800 × g) for 15 min at 4 °C. The resulting supernatant was discarded and re-suspended in ice-cold buffer (2 ml homogenization buffer without BSA) and stored (Zhang et al., 2018). The cDNA of the mitochondrial was synthesized and expression of main mitochondrial genes were measured with specific sequences primers that include; COX-1 5‘-3‘F: ATCACTACCAGTGCTAGCCG and 5‘-3‘R: CCTCCAGCGGGATCAAAGAA, COX-2 5‘-3‘F: ACCAGCAGTTCCAGTATCAGA and 5‘-3‘R: CAGGAGGATGGAGTTGTTGTAG, ND1 5‘-3‘F: ATTACTTCTGCCAGCCTGACC and 5‘-3‘R: GGCCCGGTTTGTTTCTGCTA, Nrf2 5‘-3‘F: TCTCCTCGCTGGAAAAAGAA and 5‘-3‘R: AATGTGCTGGCTGTGCTTTA, Cytb 5‘-3‘F: GGCTACGTCCTTCCATGAGG and 5‘-3‘R: TGGGATGGCTGATAGGAGGT, and Actb as internal reference gene 5‘-3‘F: AGAAGCTGTGCTATGTTGCTCTA and 5‘-3‘R: TCAGGCAGCTCATAGCTCTTC.
2.7. Lung histological
Lung tissues of the mice were taken and fixed with formalin solution, and after histological sections preparation, were stained with H&E and PAS. The Histopathological slides were evaluated under the light microscopy for Peri-bronchiolar and perivascular eosinophil inflammation, mucus hypersecretion and hyperplasia of goblet cell, using a point scoring system [as described before (Masoume Athari et al., 2018, Mehrabi Nasaba et al., 2020)].
2.8. Statistical analysis
The version 19 SPSS was performed for statistical analyses. All data were shown as the mean ± SD (of at least three independent experiments). Data were analyzed using ANOVA test and Dunnett post hoc tests. P value <0.05 was considered as the significant. The graphs were drawn in GraphPad prism (ver. 5.0).
3. Results
3.1. Balf's eosinophils
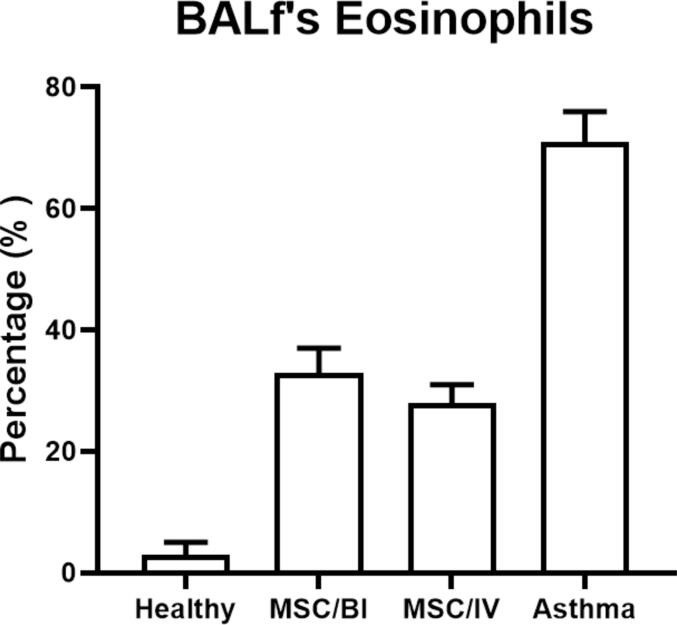
The eosinophils percentage was counted in the BALf and asthma group had increased eosinophils percentage in the BALf (71 ± 5%) compared to healthy group on days 31 (3 ± 2 %). Treatment with MSCs could significantly decrease eosinophil percentage (IT: 33 ± 4% and IV: 28 ± 3%) on day 31 (P < 0.05) (Fig. 1).
Fig. 1.
Eosinophils in BALf. Slides of the BALf were prepared and stained. Then, eosinophil percentage in BALf slides was determined.
3.2. Cytokines
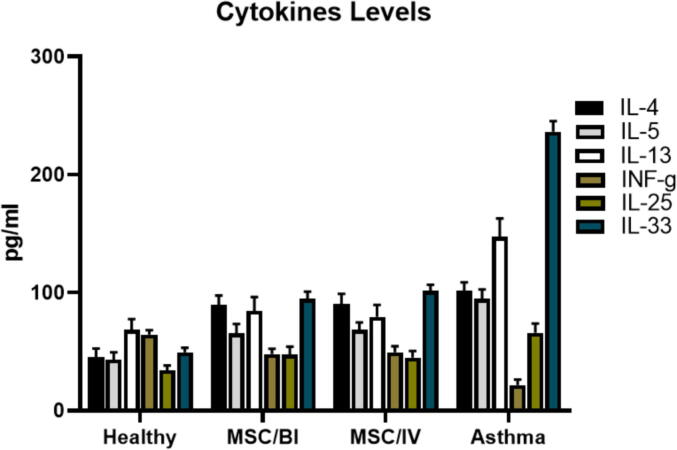
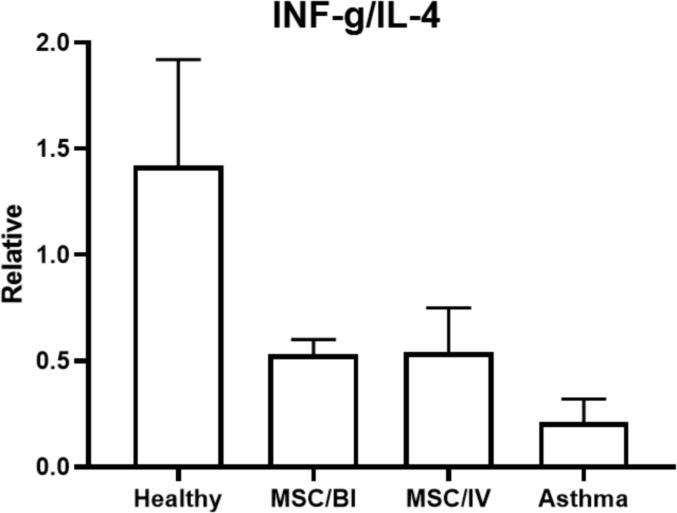
The levels of IL-4, −5, −13, −25 and –33 were significantly increased in the asthma group compared with healthy animals and a reverse trend was found in IFN-γ (P < 0.05). MSC/BI and MSC/IV treatments significantly decreased IL-4 (89.5 ± 8.0 and 90.4 ± 8.4 pg/mL respectively), IL-5 (65.4 ± 7. 9 and 68.4 ± 6.3 pg/mL respectively), IL-13 (84.5 ± 11.7 and 79.5 ± 9.9 pg/mL respectively), IL-25 (47.4 ± 6.8 and 44.9 ± 5.6 pg/mL respectively), and IL-33 (94.6 ± 6.2 and 101.3 ± 5.3 pg/mL respectively) levels in BALf (P < 0.05) and significantly (P < 0.05) increased IFN-γ level (47.9 ± 4.6 and 49.3 ± 5.2 pg/mL respectively) (Fig. 2). Also, the decreased INF-γ/IL-4 ratio in asthma group, was increased by MSC/BI and MSC/IV treatments (Fig. 3).
Fig. 2.
Cytokines. After sampling of BALf, the levels of IL-4, −5, −13, −25, –33, and IFN-γ were measured in BALf of the mice.
Fig. 3.
IFN-γ/IL-4 ratio. After sampling of BALf, the levels of IL-4 and IFN-γ were measured in BALf.Then the ratio of IFN-γ/IL-4 was calculated.
3.3. Eicosanoid
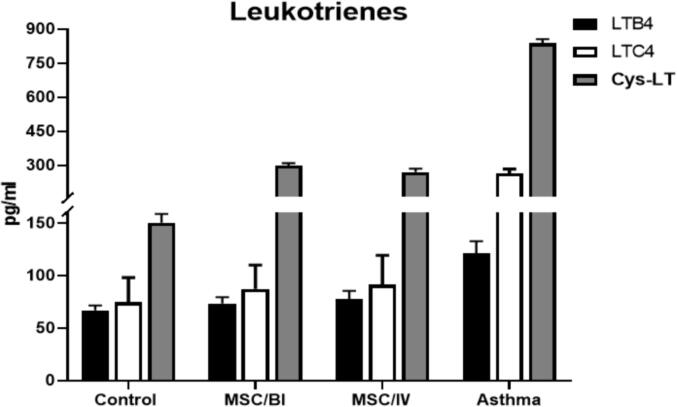
The levels of LTB4, LTC4 and Cys-LT were significantly increased in asthma mice compared too healthy mice (p < 0.05). Treatment with MSC/BI and MSC/IV significantly decreased the levels of LTB4 (73.5 ± 5.9 and 77.4 ± 8.0 pg/ml respectively), LTC4 (87.5 ± 22.5 and 91.2 ± 28.1 pg/ml respectively) and Cys-LT (298.5 ± 11.3 and 270.6 ± 14.3 pg/ml respectively). There was no significant difference (p > 0.05) between MSC/BI and MSC/IV treatments in the amount of LTB4, LTC4 and Cys-LT (Fig. 4).
Fig. 4.
Leukotrienes. The levels of LTB4, LTC4, and Cys-LT were measured in all studied groups.
3.4. Gene expression
Mitochondria genes have important role in the asthma pathology, and the main mitochondria genes were evaluated in this study. The expression of COX-1, COX-2, Cytb and ND-1 genes were increased in asthma compared to healthy mice and reverse trend was found about Nrf-2. Treatment with MSCs via IT and IV could significantly (p < 0.05) decrease gene expression of COX-1 (1.4 ± 0.3 and 1.3 ± 0.2 respectively), COX-2 (7.5 ± 1.5 and 7.7 ± 1.4 respectively), Cytb (1.8 ± 0.2 and 1.5 ± 0.3 respectively) and ND-1 (1.2 ± 0.2 and 1.4 ± 0.1 respectively) and increase Nrf-2 (0.7 ± 0.2 and 0.7 ± 0.2 respectively) but no significantly (p > 0.05) (Fig. 5).
Fig. 5.
Mitochondrial Gene expression. The gene expressions of ND-1, Nrf-2, COX-1 and −2 and Cytb were assessed in all groups by real-time PCR.
3.5. Histopathology
Eosinophilic inflammation in perivascular and peribronchial, hyper-production of mucus and hyperplasia of the goblet cell were significantly increased in non-treated asthma mice compared with healthy mice (p < 0.05). Perivascular and peribronchial eosinophilic inflammation, mucus over-production and goblet cell hyperplasia were significantly decreased (p < 0.05) in MSCs treatment via IT (1.7 ± 0.3, 1.6 ± 0.4, 2.9 ± 0.1, 2.7 ± 0.3, respectively) and IV (1.9 ± 0.2, 1.5 ± 0.5, 2.6 ± 0.3, 3.0 ± 0.1, respectively) (Fig. 6).
Fig. 6.
Lung histopathology. Lung sections were stained with H&E and PAS and then, the infiltration of eosinophils around perivascular and peribronchial, goblet cell hyperplasia, and mucus secretion were studied in the lung and were shown with yellow arrow, red arrow, black arrow and blue arrow respectively.
3.6. Discussion
Asthma is a chronic lung disease with airway obstruction, inflammation and remodeling. Although current anti-asthma therapies are poorly effective in reducing inflammation. New therapeutic options for asthma with fewer side effects, stable therapeutic and inhibited pathological changes in the lungs are required. MSCs are promising for the novel therapies development in regenerative medicine and therapeutic modality in inflammatory diseases. MSCs have more plasticity and can able to differentiate into bronchial, alveolar, vascular and interstitial cell types (Firinci et al., 2011, Parekkadan et al., 2008). Since, there were no enough studies about the effect of MSCs on the asthma, thus, we aimed to investigate the efficacy of MSCs especially on remodeling in murine model of chronic asthma.
MSCs play more relevant role in modulating of local inflammation in lung and help to repair the lung tissue and also intra-tracheal administration of MSCs decrease lung injury. It can also decrease pulmonary edema TNF-α and MIP-1b in the bronchoalveolar lavage. When MSCs are administrated systemic rout MSCs reduce the pro-inflammatory cytokines MMP-2 and −9 levels in the lungs [1113]. In our study systemic and intra-tracheal administration of MSCs could significantly reduce the levels of IL-33 25–13 −5 and reverse trend about INF-γ. But there was no significant difference between systemic and intra-tracheal administration of MSCs in reduction of IL-33 25–13 −5 levels or increasing of INF-γ level. INF-γ/IL-4 ratio shows the Th1/Th2 balance and when INF-γ/IL-4 is high Th1/Th2 is balance and main response is Th1 dominant. Therefore Th2 response was suppressed and allegro-inflammatory output was controlled. In this study increased INF-γ/IL-4 ratio by MSCs therapy showed that treatment had effect on control of Th2 responses. Also there was no significant difference in ratio INF-γ/IL-4 between systemic and intra-tracheal administration of MSCs
MSCs as self-renewing and multi-potent progenitor cells have more plasticity and the immune-modulatory potentially approaches for the control of allergic asthma and airways inflammation in the lung. Although MSCs have beneficial effect on the lung regeneration and repair. It was showed that IV administration of MSCs contributed to migration of fibroblasts and myofibroblasts in damaged lung [61114]. Systemic administration of MSCs suppress airway remodelling reduce goblet cells number collagen deposition the response of airway to methacholine. The MSCs can suppress Th2-driven allergic immune responses in ragweed-induced asthma (Ogulur et al., 2014). In this study IV and IT administration of MSCs could harness eosinophilic infiltration in BALf and also these treatment could decrease eosinophilic inflammation around bronchi and vessels of the asthmatic lung. IV and IT administration of MSCs reduced producing of mucus and hyperplasia of goblet cells in asthmatic mice. Producing of mucus was reduced better in IV than IT administration of MSCs and reduction of the goblet cells hyperplasia was controlled better in IT than IV administration of MSCs. Moreover any adverse effects were not observed in histopathological sections of the MSCs administration groups
Recent studies have suggested that in asthma, the immune-modulatory effect of MSCs is attributed to the soluble agents' secretion rather than their differentiation potential (Zachar et al., 2016, Zhang et al., 2014, Kapoor et al., 2012). (Lin et al., 2018) study showed that MSCs-derived from iPSCs modulate the Th2-mediated immune response in asthma. It ables to suppress allergic inflammation via Th2 cells and eosinophils modulating, and reversed the T-reg cells reduction. Immunomodulation by MSC is regarded as a novel therapeutic approach for a variety of immuno-allergic diseases due to their anti-inflammatory and also immune-privileged potential. The iPSC-MSCs with immune-modulatory effects treat the allergic airway inflammation with inhibition of inflammatory cell infiltration, Th2 cytokines, and IgE levels. Moreover, MSCs exert the immunomodulation via the secretion and inhibition of the soluble molecules including prostaglandins, CCL11, IL-33 and etc. (Lin et al., 2018, Uccelli et al., 2008, Park et al., 2010). MSCs therapy via intra-venus and intra-trachea could decrease main leukotrienes LTB4, LTC4 and Cys-LT that have important role in allergic asthma pathophysiology and airway inflammation. Both IV than IT administration of MSCs had similar effect in reduction of LTB4, LTC4 and Cys-LT and decreasing of allegro-inflammatory response in lung.
Mitochondria is important organelles in oxidative stress and mitochondrial dysfunction, genes encoding mitochondrial dynamics, Cytochrome oxidase activity and ATP production are linked to inflammatory lung disease. Mitochondria are the critical sites of energy and oxidative phosphorylation are regulated by nucleus genes and also mitochondrial genes for example Nrf1 and 2, TFAM, MFN1 and 2, OPA1, FIS1, ND1, Drp1, COX-1 and 2, Cytb and c. Mitochondrial dynamics and biogenesis directly impact function, resulting in an expression of related genes, alter mitochondrial enzymatic activities and control energy metabolism. In addition, mitochondrial biogenesis is strongly controlled by PGC1a, TFAM, Nrf1 and 2 and the function is introduced by COX-1 and 2, Ctyb and c (Chung et al., 2017, Trevisan et al., 2018). The Nrf2 has a pivotal role in the oxidative stress response and Nrf2 involves in the pathogenesis of the respiratory diseases. Nrf2 activation is a potential therapeutic strategy for allergic asthma and can suppress lung inflammation caused by eosinophils (Nagashima et al., 2019, Liu et al., 2019). Furthermore, we found that IV and IT administration of MSCs regulated the expression of COX-1 and 2, Cytb and ND1 and also increased the expression of Nrf2 as protective factor against inflammation and asthma. The mitochondrial biogenesis and dynamics regulation occurred at transcriptional levels. MSCs interference the expression of key genes of the mitochondria and with managing of these genes, have main force in control of gene expression and inflammatory response in injured airway.
MSC /BI and MSC/IV treatments significantly decreased BALf's eosinophils the levels of allergo-inflammatory cytokines (IL-4–5 −13–25 –33) leukotrienes (LTB4 LTC4 Cys-LT) the mitochondria genes expression (COX-1 COX-2 Cytb and ND-1) perivascular and peribronchial inflammation mucus hyper-production and hyperplasia of the goblet cell in pathological sections and reverse trend was found about Nrf-2 gene expression IFN-γ level and ratio of the INF-γ/IL-4. There was no significant difference between MSC/BI and MSC/IV treatments in the changing of these bio-factors and both treatments (MSC/BI and MSC/IV) had similar effect on control of allergic asthma pathophysiology. It may be observed that the rout of MSCs administration is not very important and the efficacy of MScs is notable. Also effecting time of MSCs in two routs (BI and IV) may be different that was not evaluated in this study. Interestingly we found that the MSCs therapy can control inflammation and immune-inflammatory factors in asthmatic lung and with controlling of mitochondrial signaling pathways and related genes prevent lung damage and asthma immune-pathology. Our results corroborated immune-regulatory and immune-modulatory effect of MSCs on the allegro-inflammatory immune response in airway of the asthmatic lung.
There were some limitations. Staining of histophatological section for collagen precipitation study and also, remodeling related bio-factors were not done. The effect of MSCs on chronic form of airway inflammation and asthma was not studied.
Ethical Approval
All methods of study on the mice were approved by the ethic committee of animal house of ix.med.vet.dep, 2021 (No. IX.MED.VET.DEP.REC.2021.290048.4).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Seyyed Shamsadin Athari, Seyyede Masoume Athari, Fateme Beyzay, Masoud Movassaghi, Esmaeil Mortaz, Mehdi Taghavi. Critical role of Toll-like receptors in pathophysiology of allergic asthma. European Journal of Pharmacology 2017; 808:21-27. [DOI] [PubMed]
- Masoume Athari S., Mehrabi Nasab E., Shamsadin Athari S. Study effect of Ocimum basilicum seeds on mucus production and cytokine gene expression in allergic asthma mice model. Revue française d’allergologie. 2018;58(7):489–493. [Google Scholar]
- Mehrabi Nasaba E., Athari S.M., Motlagh B., Athari S.S. Effects of oral administration of Ocimum basilicum on goblet cell hyperplasia and upstream cytokine gene expression in allergic asthma. Revue française d’allergologie. 2020;60(2):64–68. [Google Scholar]
- Lin T.-K., Liou C.-W., Chen S.-D., Chuang Y.-C., Tiao M.-M., Wang P.-W., Chen J.-B., Chuang J.-H. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson's disease. Chang Gung Med. J. 2009;32(6):589–599. [PubMed] [Google Scholar]
- Young Min Cho, Ju Han Kim, Mingoo Kim, Su Jin Park, Sang Hyeok Koh, Hyo Seop Ahn, Gyeong Hoon Kang, Jung-Bin Lee, Kyong Soo Park, Hong Kyu Lee. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One 2012; 7(3):e32778. [DOI] [PMC free article] [PubMed]
- Ismail Ogulur, Gulben Gurhan, Ayca Aksoy, Gokhan Duruksu, Cigdem Inci, Deniz Filinte, Faruk Erdem Kombak, Erdal Karaoz, Tunc Akkoc. Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma. International Immunopharmacology 2014; 20(1):101–109. [DOI] [PubMed]
- Yan Z., Zhuansun Y., Chen R., Li J., Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Experim. Cell Res. 2014;324(1):65–74. doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Jian-guo L.i., Yong-xun Z.-S., Wen Bing W.u., Hao H.-T., Hridayabibhu G., Pi-xin R. Human mesenchymal stem cells elevate CD4+CD25+CD127low/-regulatory T cells of asthmatic patients via heme oxygenase-1. Iranian J. Allergy, Asthma, Immunol. 2013;12(3):228–235. [PubMed] [Google Scholar]
- Huang S., Xu L., Sun Y., Wu T., Wang K., Li G. Liangliang Xu, Yuxin Sun, Tianyi Wu, Kuixing Wang, Gang Li. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Journal of Orthopaedic. Translation. 2015;3(1):26–33. doi: 10.1016/j.jot.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dash RK, Jacobs ER, Camara AKS, Clough AV, Audi SH. Integrated computational model of the bioenergetics of isolated lung mitochondria. PLoS ONE 2018; 13(6):e0197921. [DOI] [PMC free article] [PubMed]
- Fatih Firinci, Meral Karaman, Yusuf Baran, Alper Bagriyanik, Zeynep Arikan Ayyildiz, Muge Kiray, Ilknur Kozanoglu, Osman Yilmaz, Nevin Uzuner, Ozkan Karaman. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. International Immunopharmacology 2011; 11(8):1120–1126. [DOI] [PubMed]
- Parekkadan B., Tilles A.W., Yarmush M.L. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26(7):1913–1919. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- Zachar L., Bacenkova D., Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yin Y., Lai R.C., Tan S.S., Choo A.B.H., Lim S.K. Mesenchymal Stem Cells Secrete Immunologically Active Exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- Kapoor S., Patel S.A., Kartan S., Axelrod D., Capitle E., Rameshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy–induced asthma. J. Allergy Clin. Immunol. 2012;129(4):1094–1101. doi: 10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Lin Y.-D., Fan X.-L., Zhang H., Fang S.-B., Li C.-L., Deng M.-X., Qin Z.-L., Peng Y.-Q., Zhang H.-Y., Fu Q.-L. The genes involved in asthma with the treatment of human embryonic stem cell-derived mesenchymal stem cells. Mol. Immunol. 2018;95:47–55. doi: 10.1016/j.molimm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Park H.-K., Cho K.-S., Park H.-Y., Shin D.H., Kim Y.-K., Jung J.S., Park S.K., Roh H.J. Stem Cells Dev. 2010;19(11):1811–1818. doi: 10.1089/scd.2009.0513. [DOI] [PubMed] [Google Scholar]
- Chung E., Joiner H.E., Skelton T., Looten K.D., Manczak M., Reddy P.H. Maternal exercise upregulates mitochondrial gene expression and increases enzyme activity of fetal mouse hearts. Physiol. Rep. 2017;5(5):e13184. doi: 10.14814/phy2.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatiana Trevisan, Diana Pendin, Aldo Montagna, Sergio Bova, Anna Maria Ghelli, Andrea Daga. Manipulation of Mitochondria Dynamics Reveals Separate Roles for Form and Function in Mitochondria Distribution. Cell Reports 2018; 23(6):1742–1753. [DOI] [PubMed]
- Nagashima R., Kosai H., Masuo M., Izumiyama K., Noshikawaji T., Morimoto M., Kumaki S., Miyazaki Y., Motohashi H., Yamamoto M., Tanaka N. Nrf2 Suppresses Allergic Lung Inflammation by Attenuating the Type 2 Innate Lymphoid Cell Response. J. Immunol. 2019;202(5):1331–1339. doi: 10.4049/jimmunol.1801180. [DOI] [PubMed] [Google Scholar]
- Qinmei Liu, Yun Gao, and Xinxin Ci. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Medicine and Cellular Longevity 2019, Article ID: 7090534. [DOI] [PMC free article] [PubMed]