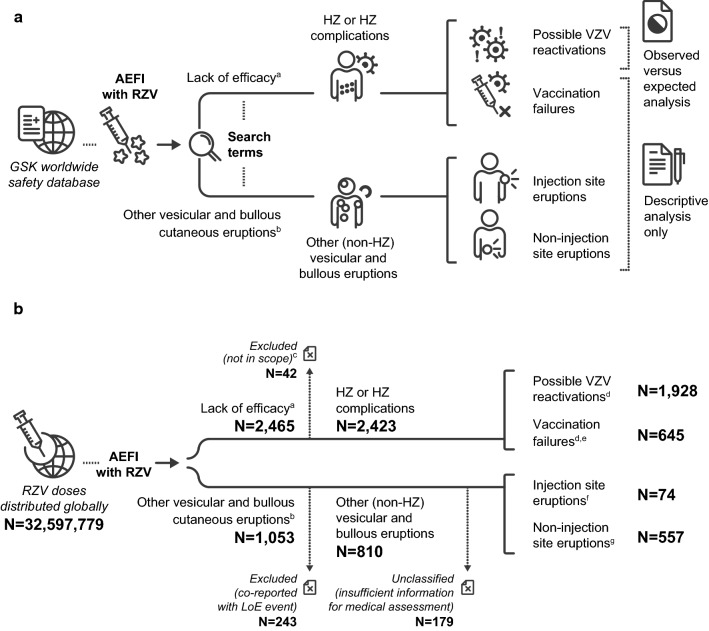
Fig. 1.
a Study methodology and b number of reports identified. Note: One report could contain more than one adverse event reported by the same individual. aIdentified with the Medical Dictionary for Regulatory Activities (MedDRA®) preferred terms listed in ESM 2. bIdentified with the MedDRA® preferred terms listed in ESM 3. cExcluded because linked to a co-administered vaccine and not to the adjuvanted recombinant zoster vaccine. dReports with unknown time to onset were included among both “possible VZV reactivations” and “vaccination failures.” eIncluded suspected and confirmed vaccination failures. fDetails on these reports are provided in ESM 6. gDetails on these reports are provided in Table 2. AEFI adverse events following immunisation, ESM electronic supplementary material, HZ herpes zoster, LoE lack of efficacy, N total number of reports in a given category, RZV adjuvanted recombinant zoster vaccine, VZV varicella zoster virus