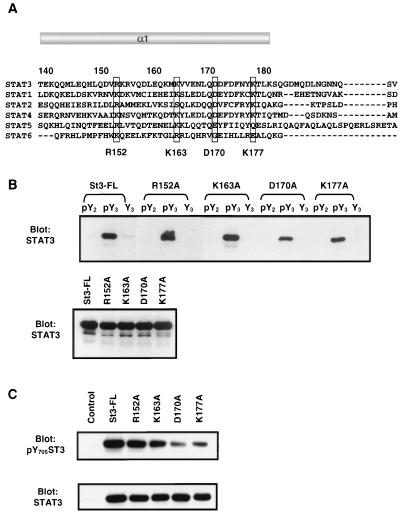
FIG. 5.
Point mutant Asp170 of Stat3 diminishes its peptide-binding activity and the tyrosine phosphorylation stimulated by EGF. (A) Sequence alignment of helix α1 in the coiled-coil domain of STATs. The highly conserved hydrophilic residues are boxed and indicated at the bottom. (B) COS-1 cells were transfected with point mutants of Stat3 as labeled on top of the upper panel. The peptide-binding experiments were performed as described in the legend to Fig. 4. The expression of the mutant Stat3 proteins is examined in Western blot analysis in the lower panel. (C) COS-1 cells were transfected with control vector or Stat3 plasmids as labeled on top of the upper panel and induced by EGF for 15 min. The cell lysates were subjected to Western blot analysis with antibodies against phospho-Tyr-705-Stat3 (upper panel) or Stat3 (lower panel).