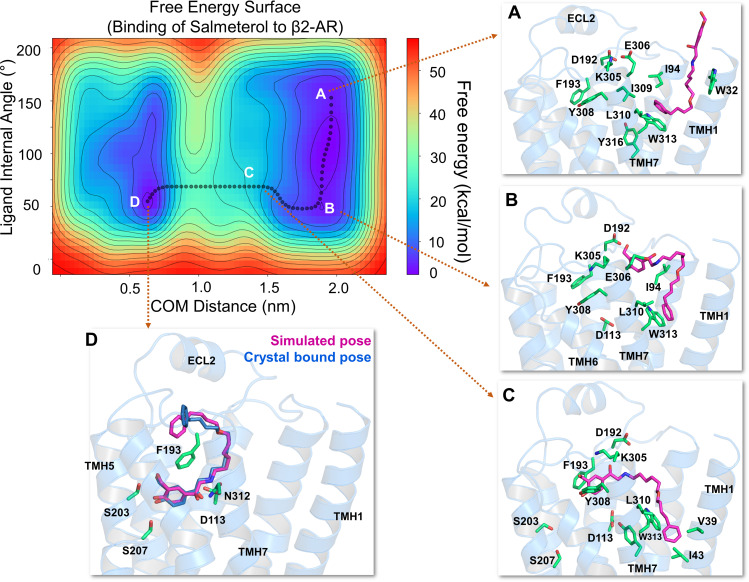
Fig. 2.
The free energy surface for salmeterol’s access and binding to the β2-adrenergic receptor through the TMHs 1 and 7 from its energetically favorable location in the membrane. The two-dimensional free energy surface is characterized by the distance between the COM of the ligand and binding site residues (x-axis) in nm and the internal angle of the salmeterol molecule (y-axis) in degrees. The minimum energy path, as determined by well tempered metadynamics simulations, is given in bold black line, and several representative intermediate states along the path were labeled (A–D). (A) As salmeterol (licorice representation, magenta color) approaches the receptor from within the membrane, its aryl-alkoxy-alkyl tail comes into contact with the hydrophobic residues, including W3137.40, I3097.36, L3107.37, V391.38, W421.41, and I431.42 from TMHs 1 and 7. (B and C) Salmeterol undergoes a significant conformational change from its extended to bent form and engages in H-bond interactions with D192ECL2 through its O1 and O2 groups, which destabilize the salt bridge between D192ECL2 and K3057.32. The breakage of the salt bridge allows salmeterol to move further, disrupting the hydrophobic lock (between F193ECL2 and Y3087.35) while the tail is still anchored by the hydrophobic residues. (D) Outward movement of the side chain indole ring of W3137.40 releases the tail and facilitates salmeterol’s entry into the pocket, which then assumes its final bound pose, mostly similar to that of the crystal structure pose (PDB ID 6MXT) (Masureel et al., 2018). In its final bound pose, salmeterol engages in polar H-bond and salt bridge interactions with S2035.42, S2075.46, D1133.32, and N3127.39. Salmeterol (crystal pose in dark blue) and the binding site residues (green) are illustrated in licorice representation. The receptor is illustrated in secondary structure representation (light blue).