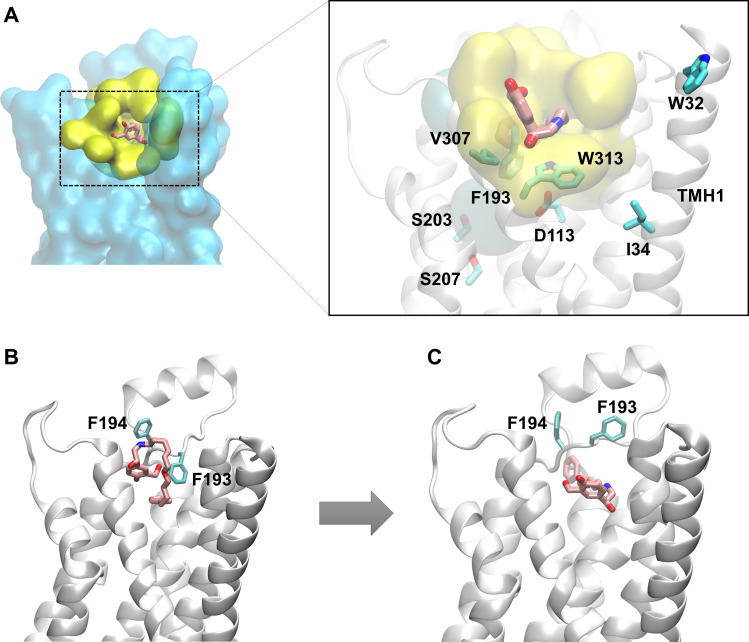
Fig. 3.
Salmeterol enters the β2-AR binding site from within the membrane through the transmembrane helices 1 and 7 using its lipophilic tail as a passkey. (A) Hydrophobic residues from TMHs 1 and 7 anchor the lipophilic tail of salmeterol and facilitate salmeterol’s entry into the pocket from within the membrane. (B) However, in several simulations, salmeterol’s entry by its saligenin head is blocked by the side chain phenyl ring of F193ECL2 that acts as a gate. (C) Fascinatingly, salmeterol flips 180° and presents its aryl-alkyl tail as a passkey to the gate, which immediately opens up the channel by flipping F193ECL2 upward to gain entry into the pocket.