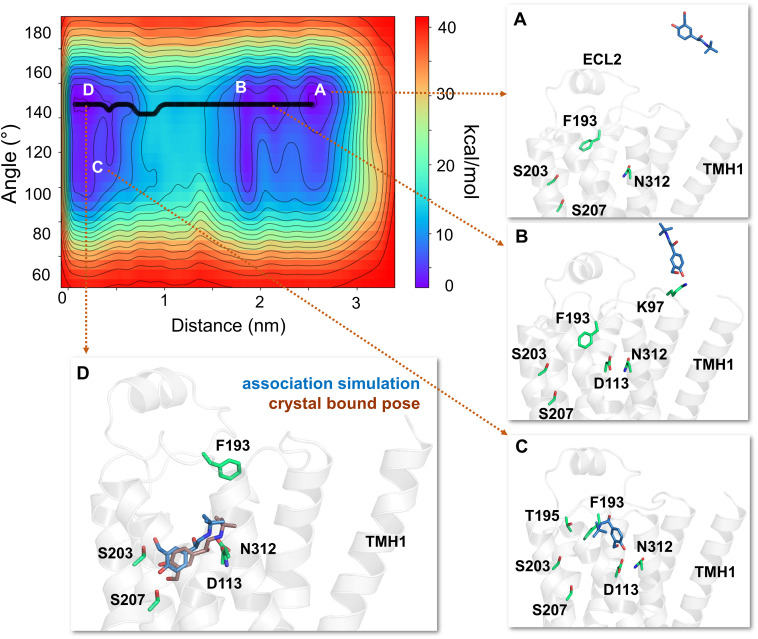
Fig. 5.
The free energy surface and minimum energy path for salbutamol’s access and binding to the β2-adrenergic receptor. The primary binding path for salbutamol is through the common aqueous route, as determined by well tempered metadynamics simulations (minimum energy path, in bold black line). (A) In each simulation, salbutamol first entered the aqueous bulk before it established its first contact with the receptor. (B) Salbutamol first encountered K972.68 (through its saligenin hydroxyl groups). (C) Salbutamol slid along the extracellular vestibule, establishing interactions with F193ECL2 through its t-butyl end and with D1133.32 and N3127.39 with its saligenin end. (D) Salbutamol is seen in its final bound pose, very similar to its crystal X-ray structure pose (RMSD of 2.25Å) bound to turkey β1-AR (PDB ID 2Y04) (Warne et al., 2011). In its final bound pose, salbutamol engages in polar interactions with S2035.42, S2075.46, D1133.32, and N3127.39. Salbutamol (blue) and the binding site residues (green) are illustrated in licorice representation. The receptor is illustrated in secondary structure representation (light pink).