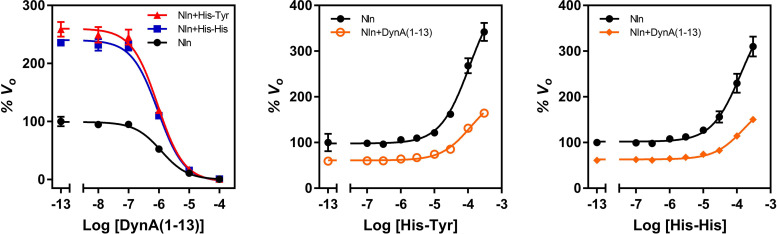
Fig. 7.
Reciprocal effects of His-Tyr or His-His and competitive inhibitor dynorphin A (1-13) on activity of Nln. Left panel presents concentration-dependent inhibitory effect of dynorphin A (1-13) on hydrolysis of synthetic substrate (15 µM) by recombinant rat Nln (0.3 nM) in the absence or presence of His-Tyr or His-His (100 µM) (mean ± S.D., n = 4 independent experiments with duplicate samples for each condition). Calculated IC50 values for dynorphin A (1-13) are 1.2 µM (95% CI, 1.03–1.38 µM) in Nln, 0.92 µM (95% CI, 0.76–1.12 µM) in Nln + His-Tyr, and 0.93 µM (95% CI, 0.78–1.11 µM) in Nln + His-His. Center and right panels present concentration-dependent effect of His-Tyr and His-His on hydrolysis of synthetic substrate (15 µM) by recombinant rat Nln (0.3 nM) in the absence or presence of dynorphin A (1-13) (1 µM) (mean ± S.D., n = 4 independent experiments with duplicate samples for each condition). Calculated A50 values for His-Tyr are 109.9 µM (95% CI, 86.3 – 141 µM) and 116 µM (95% CI, 91.5 –151 µM) in the absence and presence of dynorphin A (1-13), respectively. Corresponding Amax values for His-Tyr are 435% (95% CI, 406%–472%) and 206% (95% CI, 193%–223%). Calculated A50 values for His-His are 132 µM (95% CI, 96.8–186 µM) and 158 µM (95% CI, 119–216 µM) in the absence and presence of dynorphin A (1-13), respectively. Corresponding Amax values for His-His are 401% (95% CI, 366%–448%) and 196% (95% CI, 181%–217%). Note that the initial velocity of hydrolysis in the absence of any ligand corresponds to 100% on the vertical axis and −13 on the horizontal axis in these panels.