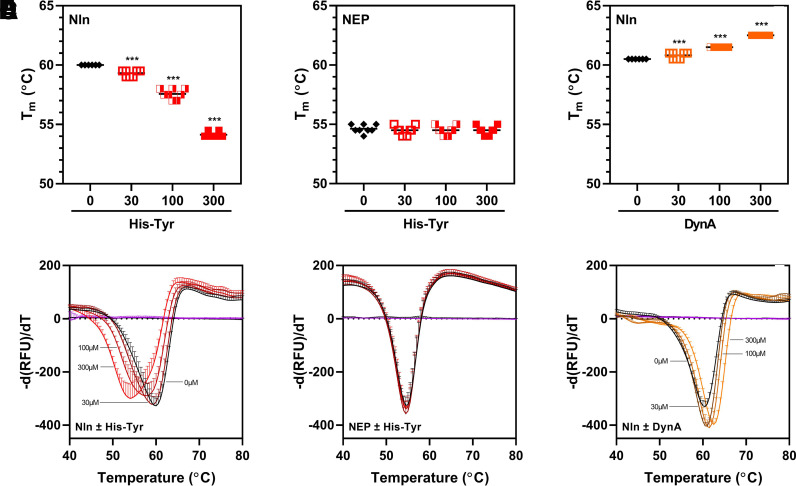
Fig. 9.
DSF analysis of His-Tyr and Nln interaction. (A and B) present concentration-dependent effect of His-Tyr (at 30, 100, and 300 µM) on midpoint of the unfolding transition (i.e., melting temperature, Tm) of recombinant Nln (A; 3 µM) and NEP (B; 3 µM). In the presence of His-Tyr, a statistically significant shift of Nln Tm toward lower temperature is observed (A), whereas NEP Tm is not affected under the same experimental conditions (B). (C) presents concentration-dependent effect of Nln inhibitor dynorphin A (1-13) (DynA, at 30, 100, and 300 µM) on Nln Tm. In this case, a statistically significant shift of Nln Tm toward higher temperature is observed in the presence of DynA [n = 4 independent experiments with duplicate samples for each condition; ***, P < 0.001 compared with a control condition wherein the respective peptidase was incubated with vehicle (0)]. The black line within the scattered dots for each experimental group indicates the mean. (D–F) Summary of negative derivative [d(RFU)/dT] curves for (A–C) are shown (RFU, relative fluorescence unit).