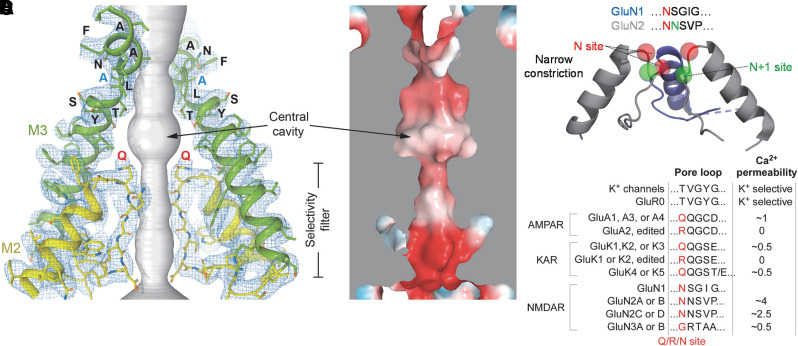
Fig. 20.
Structure of the ion permeation pathway. (A) Open-state structure of a homomeric GluA2 AMPA receptor (PDB: 5WEO). The ion conduction pathway is shown in gray, the pore-forming re-entrant M2 loop is in yellow, and the M3 gating helix is in green. Only two (B and D) out of four subunits are shown; the other two subunits (A and C) are removed for clarity. The blue mesh illustrates cryo-EM density. Residues in the SYTANLAAF motif, including the gating hinge Ala618 (blue) and the Q/R/N site glutamine (red), are labeled. (B) Electrostatic surface potential of the open pore with blue indicating a positive charge, red negative charge, and white neutral. (C) The re-entrant M2 pore loop for a GluN1/2 NMDA receptor is shown as a ribbon structure with the Q/R/N site Asn red, and a downstream adjacent Asn in GluN2 is green (indicated as N+1). (D) Summary of the Q/R/N site identity for iGluR subunits and qualitative assessment of Ca2+ permeability. Supplemental Table 2 summarizes measured Ca2+ permeabilities. KAR, kainate receptor. Panels C and D adapted with permission from Wollmuth (2018).