Fig. 22.
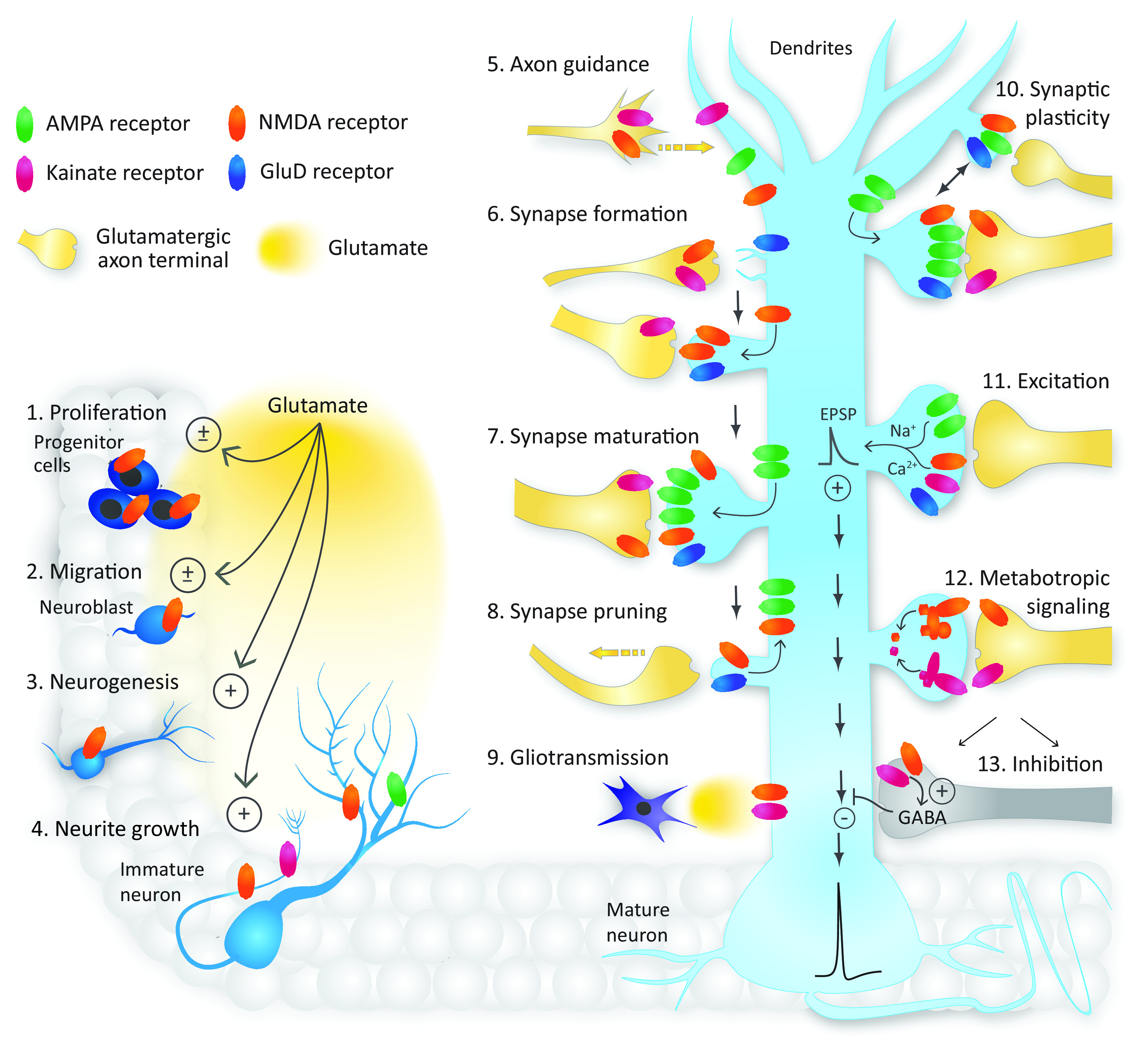
Roles of glutamate receptors in the CNS. The different iGluRs control diverse neuronal function beginning in embryonic development (left) and continuing in the mature neuron (right). NMDA receptors contribute to neuron development by regulating (1) neural progenitor cell proliferation, (2) migration, and (3) neurogenesis. NMDA receptor activation can either inhibit or enhance proliferation and migration. (4) NMDA and AMPA receptors play key roles in dendrite development, whereas NMDA and kainate receptors regulate axon growth and (5) axon guidance toward appropriate synaptic targets. (6) Synapse formation is regulated by postsynaptic GluD and NMDA receptors as well as presynaptic NMDA and kainate receptors, with kainate receptors playing a role in axon terminal maturation. (7) Synapse maturation involves the recruitment of AMPA receptors as well as continued activity of NMDA, kainate, and/or GluD receptors, whereas (8) synapse pruning involves NMDA and GluD receptors and the removal of AMPA receptors. (9) Extrasynaptic NMDA and kainate receptors regulate neuronal excitability in response to glutamate release from astrocytes. (10) Postsynaptic AMPA, NMDA, and GluD receptors have roles in short- and long-term synaptic plasticity, and NMDA and kainate receptors are implicated in presynaptic mechanisms underlying plasticity. (11) All iGluR subtypes (except GluD receptors) mediate depolarizing EPSPs that increase neuronal excitability and promote action-potential generation. (12) NMDA and kainate receptors have metabotropic signaling capabilities. (13) Presynaptic NMDA and kainate receptors are expressed at GABAergic synapses where they enhance inhibitory synaptic transmission.
