Abstract
Anterior cruciate ligament (ACL) reconstruction is an increasingly common procedure as orthopaedic patients seek to remain active longer in life, resulting in more ligamentous knee injuries. Graft selection is at the forefront of decision making in knee reconstruction, with advantages and disadvantages to various grafts, including allograft to autograft. Although the gold standard for the ACL reconstruction of elite athletes and highly active patients has traditionally been bone–patellar tendon–bone autograft (BTB), this graft is not without its disadvantages, such as increased operative time, increased postoperative pain, potential for anterior knee pain, larger incision, violation of the extensor mechanism, and potentially kneeling pain. Soft tissue autografts (hamstring, quadriceps) offer a good alternative; however, they may be associated with higher rerupture rates, as well as associated donor site morbidity. Additionally, soft tissue allografts have a higher graft rupture rate. For this reason, it is the senior author’s preference to perform allograft ACL reconstruction with BTB allograft in appropriately selected patients. We describe our technique for an efficient and reproducible BTB allograft preparation.
Technique Video
In the video, we present our preferred technique for BTB allograft preparation. Abbreviation: BTB, bone–patellar tendon–bone autograft.
Anterior cruciate ligament (ACL) reconstruction is one of the most common orthopedic procedures, with >200,000 performed annually and an incidence ranging from 30 to 78 per 100,000 people.1, 2, 3 Techniques in ACL reconstruction have evolved over the years from open procedures that restricted postoperative weightbearing and range of motion to arthroscopically assisted single-incision techniques that encouraged early range of motion and full weightbearing.4, 5, 6 Similarly, graft choice has also seen a paradigm shift. Options for graft selection include bone–patellar tendon–bone (BTB), quadriceps tendon, and hamstring autografts, as well as options such as Achilles tendon, BTB, tibialis anterior, and hamstring allografts.
Although BTB autograft is considered the gold standard for primary ACL reconstruction7, 8, 9 among high-volume ligament surgeons, particularly those who take care of intercollegiate, professional, and elite-level athletes, many factors should be considered before definitive selection. These include age, desired activity level, quality of host tissue, gender, concurrent injuries, risk of donor site morbidity, cosmesis, and desired type of graft fixation.7,10 A multitude of studies have evaluated advantages and disadvantages of each graft, but generally there is higher donor site morbidity, larger incisions, possible violation of the extensor mechanism, and increased surgical times with autografts, whereas there is a higher failure rate in patients <25 years old; an increased risk of infection, disease transmission, and cost; and reduced graft strength with allograft selections.7, 8, 9,11
BTB autograft is preferred in the senior author’s clinical practice of >35 years, especially for patients involved in high-level athletic competition; however, the role of BTB autograft versus allograft in older, less high-demand individuals is less clearly defined. The senior author experienced allograft BTB usage increasing at 5-year increments since 1986 of 1%, 3%, 13%, 24%, and >50%. Over the last 15 years, >50% of patients have been reconstructed with a BTB allograft. Additionally, in >250 revision procedures (43 by the senior author), BTB allografts were used in 75% of revisions, with a personal repeat revision rate of 3.7%.4
Overall, in our experience patients are far more likely to tear the contralateral ACL in either primary or revision situations. It is the senior author’s preference to elect for BTB allograft usage in patients who are not elite athletes, as his 25- and 30-year clinical observational experience has resulted in nearly equivalent clinical outcomes and re-tear rates in this setting.4 The aim of this paper is to describe the senior author’s preferred technique of ACL reconstruction using BTB allograft and to discuss graft preparation to ensure low failure rates. We also compare our rates of failure between autograft and allograft and demonstrate that BTB allograft is a viable and comparable option in a majority of clinical scenarios.
Surgical Technique
Preparation
For details of the surgical technique, see the Video 1. A whole low-dose-irradiated graft (2 mrad; Allosource, Denver CO) is thawed on the back table in its packaging in a bath of warm saline at the start of the case. The senior author prefers a whole BTB graft rather than a hemi-BTB graft, as the medial and lateral thirds of the patellar tendon are longer, thinner, more obliquely oriented, and mechanically inferior to the central third of the patellar tendon.12 Once thawed, the graft is inspected to ensure adequacy of the patella tendon and ensure the bone blocks are of ample quality and quantity for graft preparation and implantation. Our technique aims to re-create a graft harvest similar to our preferred autograft harvest technique: 10-mm-wide soft tissue component with a 10 × 25-mm bone plug from the patellar bone block, and an 11 × 25-mm bone block on the tibial side. We use an accessory transpatellar portal for tibial tunnel creation, with this modification allowing for predictable femoral wall placement of the femoral socket for an arthroscopic transtibial ACL reconstruction technique. This also allows for a longer femoral socket than low anteromedial drilling; thus graft–tunnel mismatch is not typically encountered. To further safeguard against this, allografts are ordered in accordance with the patient’s height, based on the literature.13
Setup
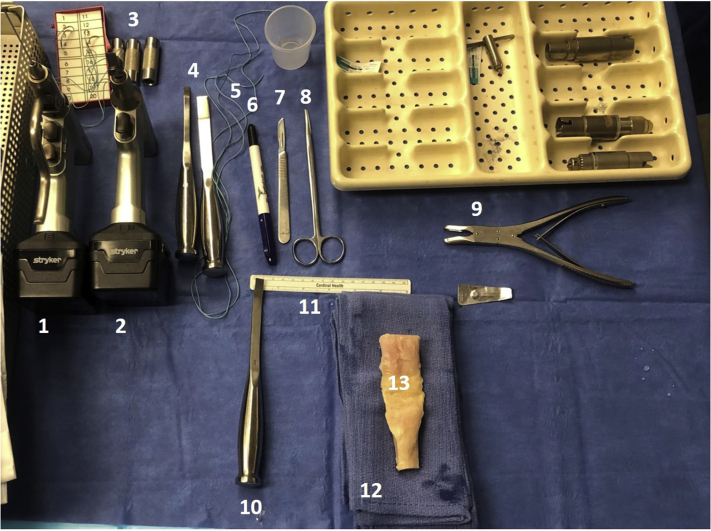
Our graft table setup is shown in Fig. 1 and includes a marking pen, #10 blade, #238 ACL saw blade, 3/8-inch osteotome, 1.6-mm Kirschner wire (K-wire), two #5 Ethibond sutures with the needles cut off, dissecting scissors, a small rongueur, and ACL sizing guides (10 and 11 mm). Once the graft is thawed, the surgeon or a skilled surgical assistant (fellow, resident, physician’s assistant, surgical technician) can begin to prepare the graft while the patient is positioned and draped, saving valuable time in the operating room.
Fig 1.
Back table setup for BTB allograft preparation. From top left to right: (1) drill with 1.6-mm Kirschner wire; (2) drill with ACL #238 saw blade; (3) ACL bone block sizing tubes; (4) osteotomes; (5) #5 Ethibond; (6) marking pen; (7) #10 blade scalpel; (8) dissection scissors; (9) small rongueur. Bottom row: (10) 3/8-inch osteotome; (11) ruler; (12) stack of towels; (13) whole BTB graft. ACL, anterior cruciate ligament; BTB, bone–patellar tendon–bone autograft.
Graft Harvest
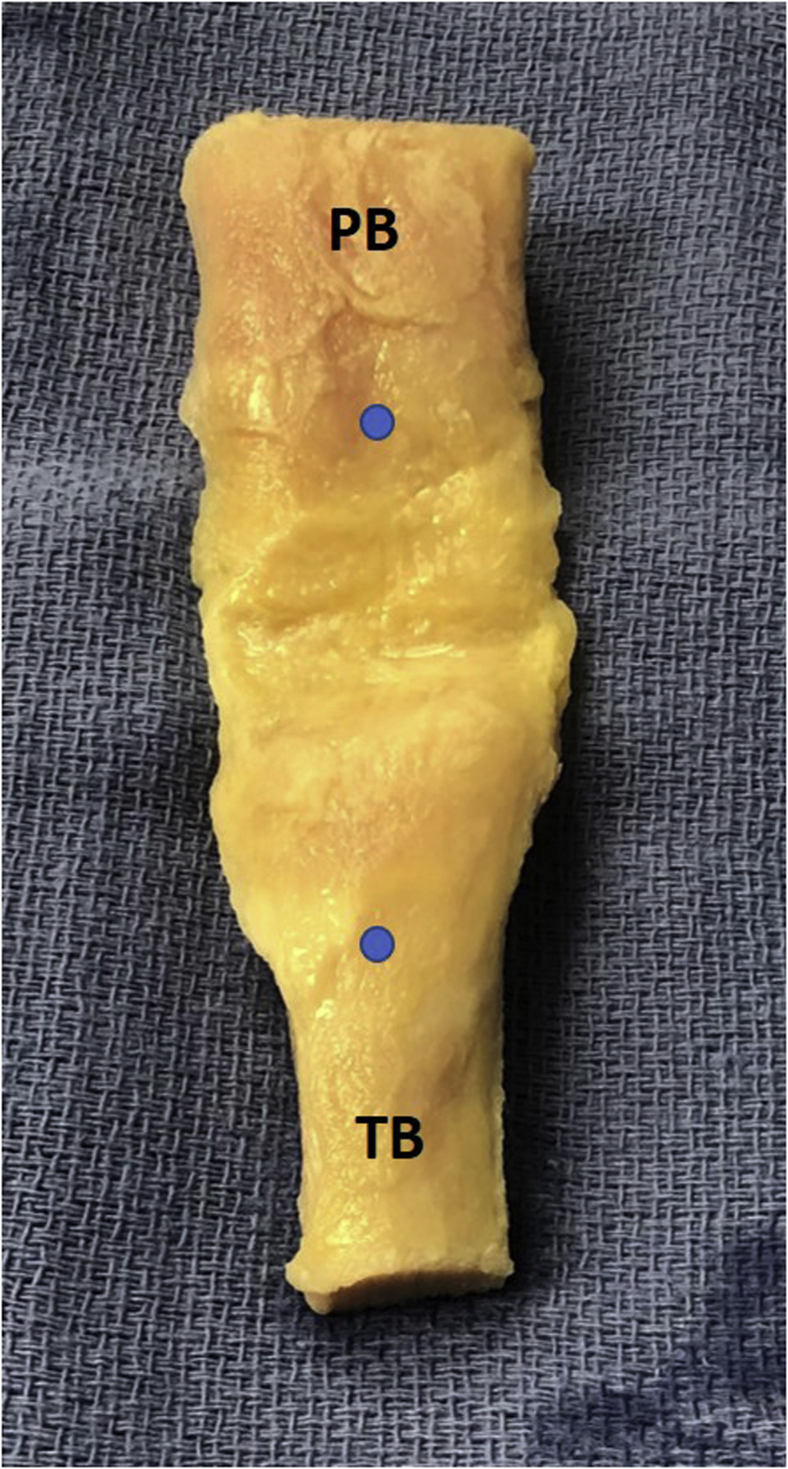
The graft is placed in an anatomic position (patella plug up, tibial plug down) on a small stack of sterile towels. A dot is placed on the inferior pole of the patella to ensure a well-centered bone block, with a corresponding dot on the tibial tubercle for cutting trajectory. A 3/8-inch osteotome (10 mm wide) and is used to size the soft tissue component of the graft on either side of the dot on the patella (Fig. 2). A #10 blade is then used, beginning on the patellar bone block and continuing through the patellar tendon, first on the righthand side, then the left, to create the 10-mm-wide graft. Bone cuts begin on the tibia, and a 25-mm-long tibial bone block is created. The vertical cut on the righthand side of the bone block is made with the saw in the surgeon’s right hand, and the saw is moved to the surgeon’s left hand to make the vertical cut on the lefthand side of the bone plug. A transverse cut is then made connecting these limbs, with the saw at an ∼45° angle to minimize past-pointing of the blade. A vertical relief cut is then made midway along the length of the transverse cut distally; this will allow for ease of bone plug removal (Fig. 3).
Fig 2.
Whole BTB graft on stack of blue towels. Graft is placed in anatomic position with the patellar plug (PB) up and the tibial plug (TB) facing down. We place a dot on the inferior pole of the patella and top of the tibial tubercle for orientation when cutting the graft, similar to a BTB autograft harvest. BTB, bone–patellar tendon–bone autograft.
Fig 3.
Schematic diagram of the planned bone cuts overlaid on the graft.
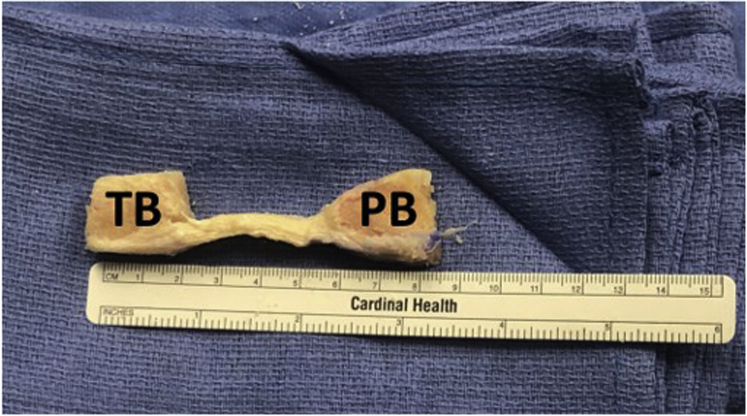
Attention is then turned to the patellar plug, which is cut in a similar fashion. A 25-mm-long bone plug is created. As this is an allograft, less attention needs to be paid to the angle of the saw blade when making the cuts; however, it is preferable to bevel the graft toward the center to save time in the later trimming of the graft. Once the 2 vertical cuts and the transverse cut have been made, the vertical relief cut is once again made approximately midway along the transverse cut, extending proximally along the remaining bone block. The osteotome can then be used to gently lever both bone plugs away from the remnant bone, producing the graft for final preparation (Fig. 4).
Fig 4.
BTB allograft after the bone and soft tissue cuts have been made. Notice the depth of the plugs: at this point, they have not yet been trimmed to size. Abbreviations: BTB, bone–patellar tendon–bone autograft; PB, patellar bone plug; TB, tibial bone plug.
Final Graft Preparation
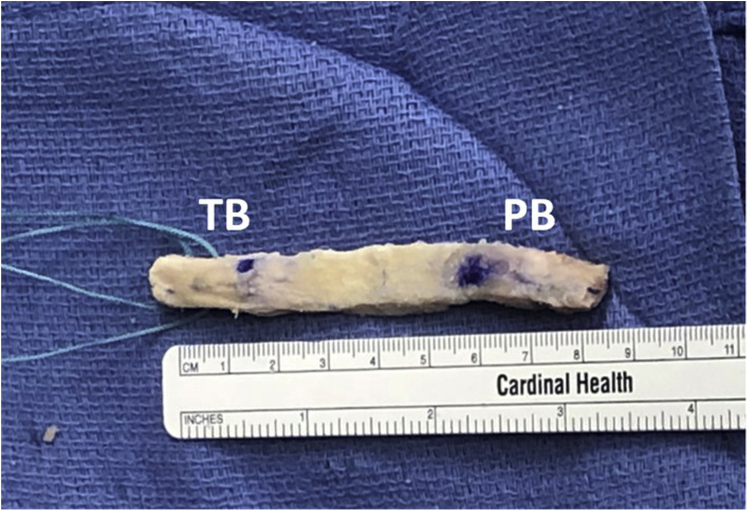
The sagittal saw is used to trim the back side of the plugs to ∼10 mm in depth. The graft should once more be inspected, ensuring 25-mm-long bone plugs on both the tibial and patellar side. A small rongueur is then used to champfer the plugs in such a manner that the patella plug fits easily within a 10-mm sizing tube and the tibial plug fits easily within an 11-mm sizing tube. Once this condition is met, a 1.6-mm K-wire is used to drill 2 holes parallel to the cortical surface of the tibial plug, and two #5 Ethibond sutures are passed through these holes and tied to one another. This will allow for tensioning of the graft on the tibial side. As the senior author uses a push-in technique rather than a pull-through technique, drill holes are not needed on the plug intended for the femoral socket. Final measurements are recorded of the length of the bone plugs as well as the length of the soft tissue component of the graft (Fig. 5). The bone–tendon interface is then marked for intra-articular identification. Lastly, the anterior cortex on the distal aspect of the tibial plug is marked so that it is easily visible, as it is the senior author’s preference to rotate the tibial plug 180° during final graft fixation to optimize graft orientation.
Fig 5.
Final graft preparation. The patella plug is up, and the tibial plug is down and contains two #5 Ethibond tagging stitches. Abbreviations: PB, patellar bone plug; TB, tibial bone plug.
Discussion
ACL reconstruction is a common procedure with generally excellent outcomes and high reproducibility. BTB autograft is the preferred graft choice for many orthopedic surgeons for ACL reconstruction owing to its strength, secure bone-to-bone healing, and accessibility.14 Nevertheless, the use of BTB allograft has become more common over the past 10 to 15 years in individuals >40 years old and those with multiligamentous injuries, poor-quality donor tissue, and radiographic evidence of degenerative joint disease.15 This is likely due to smaller incisions, a lack of donor site morbidity, shorter operative time, and the potential for less brace time because of less trauma to the extensor mechanism.7
Several studies have compared auto versus allograft. Grassi et al.11 performed a meta-analysis of 32 studies and demonstrated better outcomes with autograft as evaluated by KT-1000, revision rate, and rates of complication but reported better Lysholm and Tegner activity scores with allograft. However, when irradiated allografts were excluded, there was no longer any difference observed between the 2 groups for any metric.11 It is the senior author’s preference to use nonirradiated grafts, as irradiated grafts have been shown to have inferior structural and mechanical properties. Yao et al.16 performed a systematic review and found no differences between Lysholm score, Tegner score, KT-1000, Lachman test, or pivot shift when comparing BTB autograft and allograft. Failure rates were significantly different, but once again, when irradiated allograft was excluded, there was no difference between the 2 groups except for Tegner score.
The senior author’s 30-year experience, published in 2017, of >2400 ACL reconstructions, including 68% BTB autograft and 30% BTB allograft, has demonstrated relatively consistent revision rates over the years regardless of graft type, although there was a slightly higher rate of revision with allograft compared with autograft (2.7% versus 1.3%).4 Although this was statistically significant, it is unclear whether this minor difference is clinically significant. Furthermore, the difference may be explained by the fact that this cohort includes older data in which BTB allograft was used in individuals ≤30 years old with failure rates of 5.1%. It is now more commonly accepted that BTB autograft is the preferred graft selection in this younger population.10,17 When these individuals were excluded from the overall cohort, allograft revision rates (in patients >30 years old) dropped to 1.7% and were comparable to the ACL autograft cohort.
Throughout our experience, with the use of BTB allograft, we have learned several clinical pearls, specifically with regard to graft preparation (Table 1). There are also obvious advantages and disadvantages to BTB allograft versus autograft, as previously noted (Table 2). The limitations of BTB allograft include the cost compared with autograft, which some argue outweighs the difference in surgical time needed for donor graft harvesting.18 The risk of infection and disease transmission is also frequently referenced as a concern with allograft selection, although more recent studies have demonstrated that it not as significant as originally thought, owing to improved donor screening.19,20 Furthermore, access to tissue banks may be more difficult for smaller-volume surgeons, making autograft a more feasible option.
Table 1.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| A 3/8-inch osteotome is ∼10 cm in width and can be used to size the graft | Don’t narrow the soft tissue aspect of the graft when making the knife cuts |
| Cutting the graft in a similar fashion to BTB autograft harvest will allow the surgeon to use familiar techniques for graft preparation | Let the saw “do the work” when cutting; if the graft isn’t completely thawed, cutting can be difficult if you rush, possibly fracturing the bone blocks |
| The vertical relief cuts in the bone blocks allow for easy delivery of the bone plug | Use a stack of towels to avoid “plunging” |
| Pass the #5 Ethibond by hand with needle off, as the needle is usually too wide to fit through the drill holes | Avoid the use of bone crimpers, as they can fragment the allograft bone; instead, shape the plugs with the rongueur |
| Make sure the patellar plug is an easy 10 and the tibial plug is an easy 11 | Do not leave any prominent edges that can prohibit easy graft passage |
| Measure and record the plug and soft tissue lengths to avoid graft–tunnel mismatch | Make sure to request size-appropriate grafts based on patient height |
BTB, bone–patellar tendon–bone autograft.
Table 2.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| Allows for less invasive surgical incisions | Cost |
| Improved cosmesis | Graft availability |
| Graft can be prepped while patient is intubated and draped, saving time | Potential for transmission of infectious diseases |
| Avoids donor site morbidity | Lack of surgeon experience with this graft |
| Less traumatic to the extensor mechanism | Less biomechanical strength than autograft depending on sterilization techniques; we recommend nonirradiated graft. |
| Graft familiarity for BTB ACL surgeons | Higher rerupture rate based on activity level and sterilization process |
ACL, anterior cruciate ligament; BTB, bone–patellar tendon–bone autograft.
Overall, our experience demonstrates comparable revision rates when using BTB allograft compared with BTB autograft, making BTB allograft an attractive and viable option to consider in individuals >30 years old and those with less functional demand. Performing meticulous and precise graft preparation will yield a robust graft with excellent clinical outcomes in a high-quality ACL reconstruction.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.R.B. reports other, Arthrex, Smith & Nephew, Tournier, SLACK, CONMED, DJO, Ossur. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
In the video, we present our preferred technique for BTB allograft preparation. Abbreviation: BTB, bone–patellar tendon–bone autograft.
References
- 1.Sanders T., Kremers H., Bryan A., et al. Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am J Sports Med. 2016;44:1502–1507. doi: 10.1177/0363546516629944. [DOI] [PubMed] [Google Scholar]
- 2.Nordenvall R., Bahmanyar S., Adami S., et al. A population-based nationwide study of cruciate ligament injury in Sweden, 2001-2009: Incidence, treatment, and sex differences. Am J Sports Med. 2012;40:1808–1813. doi: 10.1177/0363546512449306. [DOI] [PubMed] [Google Scholar]
- 3.Gianotti S., Marshall S., Hume P., et al. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. J Sci Med Sport. 2009;12:622–627. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Riff A., Luchetti T., Weber A., et al. Thirty-year experience with ACL reconstruction using patellar tendon: A critical evaluation of revision and reoperation. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117724345. :2325967117724345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach B., Jr., Tradonsky S., Bojchuk J., et al. Arthroscopically assisted anterior cruciate ligament reconstruction using patellar tendon autograft: Five- to nine-year follow-up evaluation. Am J Sports Med. 1998;26:20–29. doi: 10.1177/03635465980260012101. [DOI] [PubMed] [Google Scholar]
- 6.Raab D., Fischer D., Smith P., et al. Comparison of arthroscopic and open reconstruction of the anterior cruciate ligament: Early results. Am J Sports Med. 1993;21:680–683. doi: 10.1177/036354659302100507. [DOI] [PubMed] [Google Scholar]
- 7.Kim H., Seon J.K., Jo A.R. Current trends in anterior cruciate ligament reconstruction. Knee Surg Relat Res. 2013;25:165–173. doi: 10.5792/ksrr.2013.25.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duquin T., Wind W., Fineberg M., et al. Current trends in anterior cruciate ligament reconstruction. J Knee Surg. 2009;22:7–12. doi: 10.1055/s-0030-1247719. [DOI] [PubMed] [Google Scholar]
- 9.Mo Z., Li D., Yang B., Tang S. Comparative efficacy of graft options in anterior cruciate ligament reconstruction: A systematic review and network meta-analysis. Arthrosc Sports Med Rehabil. 2020;2:e645–e654. doi: 10.1016/j.asmr.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busam M., Rue J., Bach B., Jr. Fresh-frozen allograft anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:607–623. doi: 10.1016/j.csm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Grassi A., Nitri M., Moulton S., et al. Does the type of graft affect the outcome of revision anterior cruciate ligament reconstruction? A meta-analysis of 32 studies. Bone Joint J. 2017;99-B:714–723. doi: 10.1302/0301-620X.99B6.BJJ-2016-0929.R2. [DOI] [PubMed] [Google Scholar]
- 12.Yanke A., Bell R., Lee A., et al. Central-third bone-patellar tendon-bone allografts demonstrate superior biomechanical failure characteristics compared with hemi-patellar tendon grafts. Am J Sports Med. 2013;41 doi: 10.1177/0363546513501780. :2521-2526. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein J., Verma N., McNickle A., et al. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: Correlation between patient height and patellar tendon length. Arthroscopy. 2010;26:643–650. doi: 10.1016/j.arthro.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari J., Bush-Joseph C., Bach B., Jr. Arthroscopic-assisted anterior cruciate ligament reconstruction using patellar tendon autograft substitution: Two-incision technique. Tech Orthop. 1998;13:242–252. [Google Scholar]
- 15.Glenn R., Bach B., Jr., Bush-Joseph C. Anterior cruciate ligament reconstruction: The Rush experience. Tech Orthop. 2005;20:396–404. [Google Scholar]
- 16.Yao L., Wang Q., Zhang L., et al. Patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Eur J Orthop Surg Traumatol. 2015;25:355–365. doi: 10.1007/s00590-014-1481-5. [DOI] [PubMed] [Google Scholar]
- 17.Ellis H., Matheny L., Briggs K., et al. Outcomes and revision rate after bone-patellar tendon-bone allograft versus autograft anterior cruciate ligament reconstruction in patients aged 18 years or younger with closed physes. Arthroscopy. 2012;28:1819–1825. doi: 10.1016/j.arthro.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Nagda S., Altobelli G., Bowdry K., et al. Cost analysis of outpatient anterior cruciate ligament reconstruction: Atuograft versus allograft. Clin Orthop Relat Res. 2010;468:1418–1422. doi: 10.1007/s11999-009-1178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg D., Robertson M., Vallurupalli S., et al. Allograft compared with autograft infection rates in primary anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92:402–408. doi: 10.2106/JBJS.I.00456. [DOI] [PubMed] [Google Scholar]
- 20.Smith A., Bach B., Jr., Bush-Joseph C. Allograft for revision ACL reconstruction: The RUSH experience. Sports Med Arthrosc Rev. 2005;13:86–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the video, we present our preferred technique for BTB allograft preparation. Abbreviation: BTB, bone–patellar tendon–bone autograft.
In the video, we present our preferred technique for BTB allograft preparation. Abbreviation: BTB, bone–patellar tendon–bone autograft.