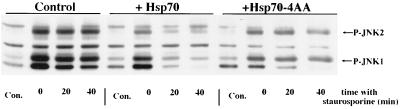
FIG. 8.
The C-terminal substitution mutant hsp70AAAA inhibits JNK activation without blocking its rate of dephosphorylation. IMR90 lung fibroblast cells (20 to 30 population doublings) were infected with an adenovirus expressing tTA (AdCMV-tTA) and an adenovirus encoding a tetracycline-regulated expression cassette encoding full-length hsp70 (+Hsp70) or hsp70 with the C-terminal four amino acids EEVD replaced with AAAA (+Hsp70-4AA). Control cells received the same total multiplicity of infection of the hsp70-encoding virus but without the Ad CMV-tTA virus (control). The infected cells were heat shocked for 30 min at 45°C and then incubated at 37°C with the protein kinase inhibitor staurosporine to block new JNK phosphorylation. Extracts were prepared from non-heat-shocked cells (Con.), heat-shocked cells (0), and heat-shocked cells that were incubated with staurosporine for 20 or 40 min at 37°C after the heat shock. Shown is an immunoblot of cytosolic extracts probed with an anti-phospho-JNK antibody.