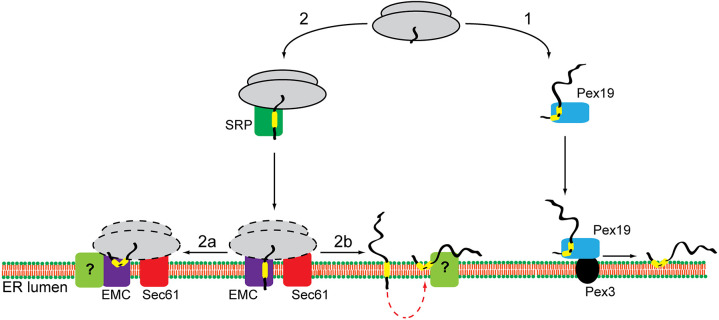
Fig. 7.
Model for LD membrane protein biogenesis at the ER. Depending on the properties of their hydrophobic membrane-inserting region, LD membrane proteins enter one of two potential biosynthetic pathways. Proteins, such as UBXD8 and HSD17B7, are released into the cytosol, bound by Pex19 and post-translationally delivered to the ER (route 1). Interaction between Pex19 and its membrane-receptor Pex3 releases such LD membrane proteins in a hairpin conformation into the ER membrane. Alternatively, LD membrane proteins, such as HSD17B11, METTL7A, METTL7B, AUP1 and HIG2, are delivered co-translationally to the ER and insert into the lipid bilayer in a reaction facilitated by the EMC with additional insertase-independent contributions from the Sec61 complex (route 2). At present, it is unclear exactly how the EMC and Sec61 complex cooperate, and which complex facilitates ribosomes binding (indicated by dashed lines). We speculate that the co-translationally delivered LD membrane proteins transiently expose their N terminus to the ER lumen but then re-arrange their topology to form a hairpin with both termini facing the cytosol, which is a prerequisite for trafficking to LDs. Such topological reorientation could occur either co-translationally (route 2a) or following complete protein synthesis (route 2b). At present, it is unknown whether this process is spontaneous or requires an assistance from a dedicated factor(s) (indicated with ‘?’).