Fig. 2.
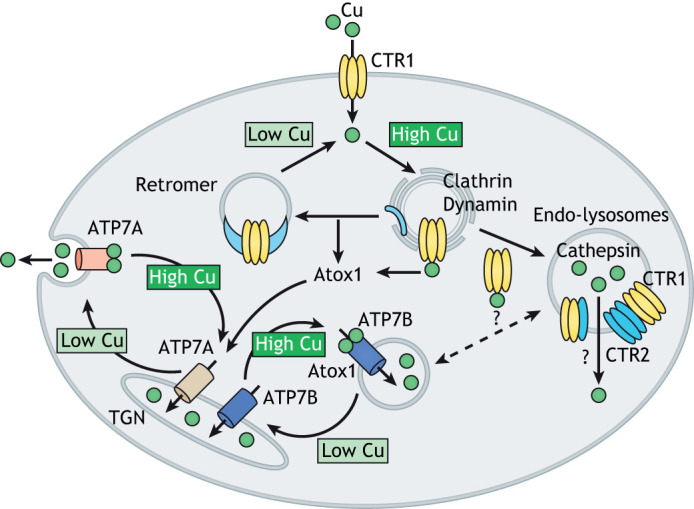
Cu-mediated regulation of the distribution of Cu transporters between cellular compartments. The amount of Cu taken into cells depends on the abundance of CTR1 at the plasma membrane. Excess Cu stimulates the clathrin- and dynamin-dependent endocytosis of CTR1. Atox1 removes Cu from the cytosolic-binding sites on CTR1 and also transfers Cu to the regulatory metal-binding sites on the ATP-driven Cu transporters ATP7A and ATP7B. From the recycling endosomes, CTR1 either returns to the plasma membrane with the assistance of retromer or moves to endo-lysosomes, where it interacts with the low-affinity Cu transporter CTR2 and regulates Cu release to the cytosol. Cu levels in the cytosol are maintained by the ATP-driven Cu transporters ATP7A and ATP7B. These transporters are typically targeted to sub-compartments of TGN but can also be found in endosomes and specialized compartments such as melanosomes. They transfer Cu from the cytosol to the TGN lumen to activate the Cu-dependent enzymes within the secretory pathway. When cytosolic Cu is high, ATP7A and ATP7B leave the TGN to facilitate Cu export (see Box 2 for details). Whether ATP7B and CTR2 move Cu in opposite direction in the same vesicular compartment is unclear.
