Abstract
Objective:
To determine whether baseline aortic stiffness, measured by aortic pulse wave velocity (PWV), relates to longitudinal cerebral gray or white matter changes among older adults. Baseline cardiac magnetic resonance imaging will be used to assess aortic PWV while brain magnetic resonance imaging will be used to assess gray matter and white matter hyperintensity (WMH) volumes at baseline, 18 months, 3 years, 5 years, and 7 years.
Approach and Results:
Aortic PWV (m/s) was quantified from cardiac magnetic resonance. Multimodal 3T brain magnetic resonance imaging included T1-weighted imaging for quantifying gray matter volumes and T2-weighted fluid-attenuated inversion recovery imaging for quantifying WMHs. Mixed-effects regression models related baseline aortic PWV to longitudinal gray matter volumes (total, frontal, parietal, temporal, occipital, hippocampal, and inferior lateral ventricle) and WMH volumes (total, frontal, parietal, temporal, and occipital) adjusting for age, sex, race/ethnicity, education, cognitive diagnosis, Framingham stroke risk profile, APOE (apolipoprotein E)-ε4 carrier status, and intracranial volume. Two hundred seventy-eight participants (73±7 years, 58% male, 87% self-identified as non-Hispanic White, 159 with normal cognition and 119 with mild cognitive impairment) from the Vanderbilt Memory & Aging Project (n=335) were followed on average for 4.9±1.6 years with PWV measurements occurring from September 2012 to November 2014 and longitudinal brain magnetic resonance imaging measurements occurring from September 2012 to June 2021. Higher baseline aortic PWV was related to greater decrease in hippocampal (β=−3.6 [mm3/year]/[m/s], 95% CI=−7.2 to −0.02, p=0.049) and occipital lobe (β=−34.2 [mm3/year]/[m/s], 95% CI=−67.8 to −0.55, p=0.046) gray matter volume over time. Higher baseline aortic PWV was related to greater increase in WMH volume over time in the temporal lobe (β=17.0 [mm3/year]/[m/s], 95% CI=7.2 to 26.9, p<0.001). All associations may be driven by outliers.
Conclusions:
In older adults, higher baseline aortic PWV related to greater decrease in gray matter volume and greater increase in WMHs over time. Because of unmet cerebral metabolic demands and microvascular remodeling, arterial stiffening may preferentially affect certain highly active brain regions like the temporal lobes. These same regions are affected early in the course of Alzheimer’s disease.
Keywords: cerebrovascular disorders, leukoencephalopathies, magnetic resonance imaging, pulse wave analysis, vascular stiffness
Subject Terms: Hypertension, Magnetic Resonance Imaging (MRI), Vascular Disease
1. Introduction
Age-related vessel wall thickening and elastin loss result in aortic stiffening, which may impair autoregulatory mechanisms1 and precede the development of hypertension.2,3 A healthy aorta is elastic and helps diffuse pressure waves leaving the heart,4 but this elasticity declines over one’s life course. As the aorta stiffens, distal cerebral microvasculature receives damaging pressure waves, eventually resulting in vessel wall damage. Aortic stiffening can even result in downstream cognitive consequences5,6 and has been shown to increase the risk of developing dementia.7
This study examined whether baseline aortic pulse wave velocity (PWV), a measure of velocity of pressure waves leaving the heart (and a proxy for arterial stiffness), related to longitudinal cerebral gray or white matter changes (separate models for each region of interest) among older adults. In the presence of high pulse pressures, cerebral microvasculature may lose its integrity8 and result in reduced brain perfusion and nutrient delivery. Reductions in perfusion drive atrophy-inducing excitotoxicity,9–11 and reduced nutrient transport to the parenchyma12,13 results in ischemia and oligemia,14 which drive white matter hyperintensity (WMH) formation.15,16 Cross-sectionally in middle-aged and older adult cohorts, increased brachial-ankle PWV,17 carotid-femoral PWV,18,19 and aortic PWV20 are associated with greater global WMHs17–21 while increased aortic PWV is correlated with lower global gray matter volume.22
We aim to build upon previous literature by assessing whether increased PWV at study enrollment contributes to neurodegeneration and white matter damage over time. We hypothesize that increased aortic PWV contributes to greater decrease in gray matter volume22 and greater increase in WMH volume over time.17 We hypothesize that these associations will be more pronounced in APOE (apolipoprotein E) ε4 carriers (given it is a vascular risk modifier)23,24 and among participants with mild cognitive impairment (MCI),20 due to greater brain homeostasis disruption, compared with participants with normal cognition (NC).
2. Material and Methods
2.1. Data Availability, Standard Protocol Approvals, Registrations, and Participant Consents
Due to participant consent restrictions in data sharing, a subset of data is available for purposes of reproducing results or replicating procedures. The data, analytic methods, and study materials can be obtained by contacting the corresponding author. The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. Written informed consent was obtained before data collection.
2.2. Study Cohort
The Vanderbilt Memory and Aging Project is a longitudinal study investigating the intersection of brain aging and vascular health in older adults.25 Participants were enrolled between September 2012 and November 2014 and seen for follow-up at 18-month, 3-year, 5-year, and 7-year timepoints. For enrollment, participants were required to be ≥60 years of age, have a reliable proxy, speak English, and have intact auditory and visual acuity to participate in the examinations. At eligibility, participants underwent an interview (including Clinical Dementia Rating26 with a proxy and assessment of activities of daily living), medical history review, and neuropsychological assessment. Participants were excluded for the following: a cognitive diagnosis other than NC, early MCI,27 or MCI;28 history of neurological disease (eg, stroke, multiple sclerosis), clinical heart failure, head injury with loss of consciousness >5 minutes, major psychiatric illness (eg, schizophrenia), or systemic or terminal illness affecting longitudinal participation; and magnetic resonance imaging (MRI) contraindication. At enrollment, participants completed a thorough evaluation, including (but not limited to) clinical interview, physical examination, echocardiogram, medication review, fasting blood draw, cardiac MR imaging, and brain MRI. Participants were excluded from the current study for missing baseline cardiac MR imaging data or for missing brain MRI data at all four epochs. For the present study, given diagnostic group comparisons, participants with early MCI27 were excluded for their small sample size (n=27). An additional 30 participants were excluded for missing or unusable predictor, outcome, or covariate data. See Figure 1 for inclusion and exclusion details.
Figure 1. Participant Inclusion/Exclusion Details.
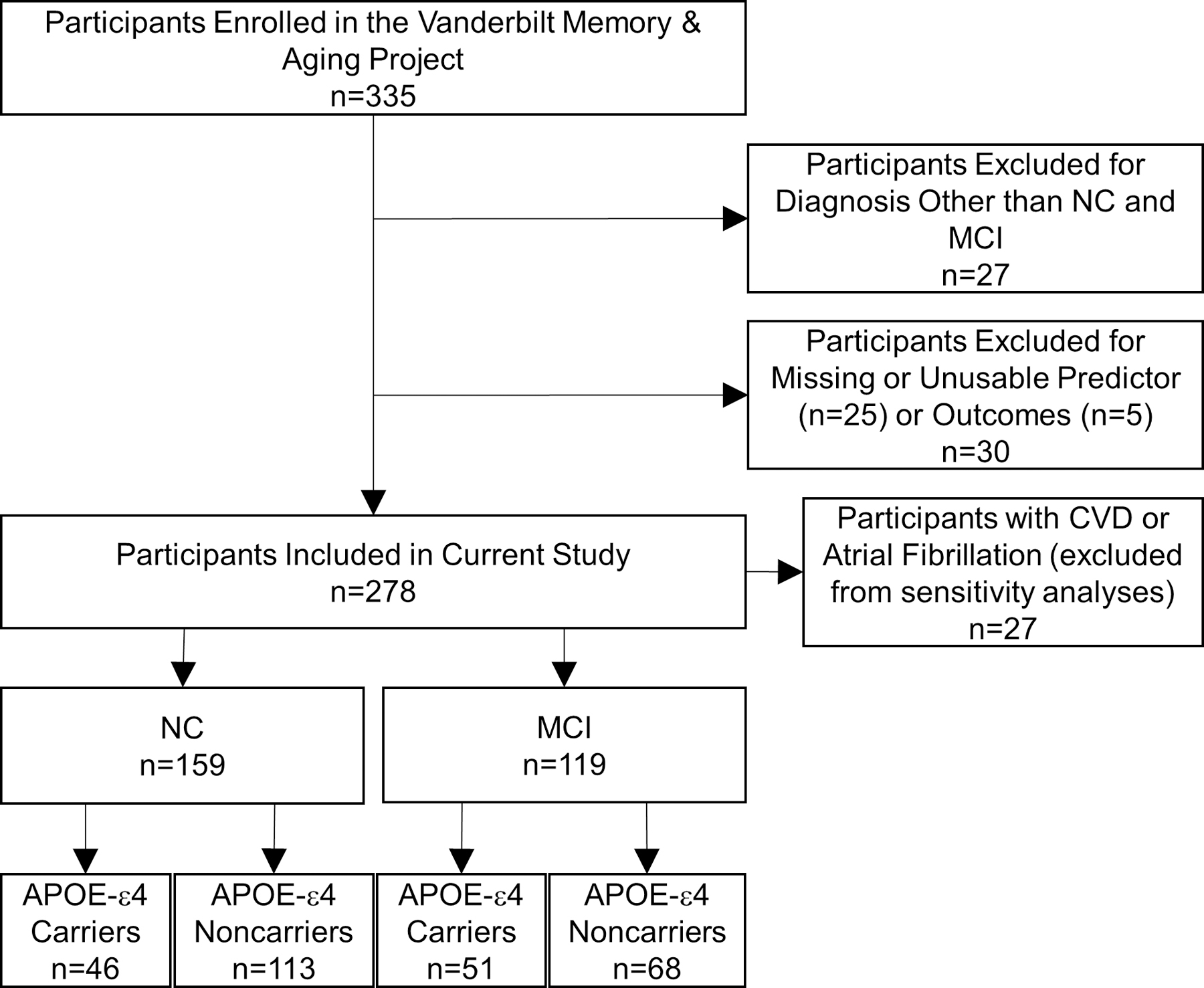
Participant inclusion and exclusion details. Missing data categories are mutually exclusive. Sensitivity analyses excluded participants with CVD or atrial fibrillation. APOE indicates apolipoprotein E; CVD, cardiovascular disease; MCI, mild cognitive impairment; and NC, normal cognition.
2.3. Aortic PWV
CMR imaging was acquired as previously described,29 using a 1.5T Siemens Avanto system (Siemens Medical Solutions, Inc., Malvern, PA) with a phased-array torso receiver coil at Vanderbilt University Medical Center. Two blinded raters (J.G. Terry and S. Nair) overseen by a board-certified radiologist (J.J. Carr) quantified velocity-encoded flow data of the ascending and descending thoracic aorta. The ascending to descending aorta centerline length (centimeter) was measured and flow transit time was calculated as the difference in time (milliseconds) between the leading edge of the ascending and leading edge of the descending aortic flow curves at half maximum. Aortic PWV (m/s) was calculated as the ascending to descending aorta centerline length (meters) divided by the transit time (seconds). Aortic PWV is the best noninvasive measure of central arterial stiffening30 because it provides a regional measurement and reduces distance measurement error30 and reliably accounts for vessel tortuosity compared with other methods.31 Interreader reliability for the PWV measurement had a coefficient of variation of 6.6% as determined by independent review of 34 scans by 2 readers (J.G. Terry and S. Nair).
2.4. Brain MRI
Between 2012 and 2017, participants were scanned at the Vanderbilt University Institute of Imaging Science on a 3T Philips Achieva system (Best, the Netherlands) with an 8-channel SENSE receiver head coil. In 2017, the system was upgraded to a 32-channel dStream head coil with digital gradient coils and new software. T1-weighted (repetition time, 8.9 ms; echo time, 4.6 ms; spatial resolution, 1×1×1 mm3) and T2-weighted fluid-attenuated inversion recovery (repetition time, 11 000 ms; echo time, 121 ms; spatial resolution, 0.45×0.45×4 mm3) images were acquired as part of the larger multimodal neuroimaging protocol. As previously described,25 T1-weighted images were post-processed using Multi-Atlas Segmentation32 and fluid-attenuated inversion recovery images were postprocessed using the Lesion Segmentation Tool toolbox for Statistical Parametric Mapping (SPM8).33 Manual labeling edits were applied based off review of individual scans and confirmed by a board-certified neuroradiologist (L.T. Davis) blinded to clinical information. Intracranial volume was calculated based on a summation of individual gray matter, white matter, and CSF volumes using T1-weighted images with SPM8.
2.5. Analytical Plan
Covariates were derived as follows: systolic blood pressure was the mean of 2 measurements. Medication review determined antihypertensive medication use. Diabetes was defined as fasting blood glucose ≥126 mg/dL, hemoglobin A1c ≥6.5%, or oral hypoglycemic or insulin medication usage. Atrial fibrillation was self-reported and subsequently confirmed by echocardiogram, cardiac MRI, documented prior procedure/ablation for atrial fibrillation, or medication use for atrial fibrillation. Left ventricular hypertrophy was defined on echocardiogram as left ventricle mass index >95 g/m2 in women or >115 g/m2 in men. Current cigarette smoking was determined by self-report of yes or no within the year before baseline. Self-report prevalent cardiovascular disease (CVD) with supporting medical record evidence included angina, coronary heart disease, or myocardial infarction (heart failure was a parent study exclusion). The Framingham Stroke Risk Profile (FSRP) score was calculated by applying points by sex for age, systolic blood pressure, antihypertensive medication use, diabetes, atrial fibrillation, left ventricular hypertrophy, current cigarette smoking, and CVD.34 APOE genotype was assessed as previously described,25 by determining the 2 single-nucleotide polymorphisms that define the ε2, ε3, and ε4 alleles. APOE-ε4 genotype was defined as positive (ε2/ε4, ε3/ε4, ε4/ε4) or negative (ε2/ε2, ε2/ε3, ε3/ε3).
Linear regression models related baseline aortic PWV to baseline gray matter (total, frontal, parietal, temporal, occipital, hippocampal, and inferior lateral ventricle volumes) and log-transformed WMH volumes (total, frontal, parietal, temporal, and occipital volumes). One region of interest variable was used per model. Mixed-effects regression models related aortic PWV at study entry to longitudinal gray matter (total, frontal, parietal, temporal, occipital, hippocampal, and inferior lateral ventricle volumes) and WMH volumes (total, frontal, parietal, temporal, and occipital volumes). One region of interest variable was used per model, resulting in 7 gray matter models and 5 WMH models. Hippocampus and inferior lateral ventricle regions were included due to their relevance in age-related atrophy35 and Alzheimer’s disease pathology.35 Inferior lateral ventricle volume was included as a gray matter variable as the inferior lateral ventricle is adjacent to regions known to atrophy first in Alzheimer’s disease, and increasing inferior lateral ventricle volume correlates with decreasing hippocampal volume.36 Volumes were summed across hemispheres. Fixed effects included baseline age, sex, race/ethnicity, education, diagnosis, FSRP (minus age), APOE-ε4 carrier status, intracranial volume, and aortic PWV, as well as time (defined as years since first brain MRI) and an interaction term for aortic PWV x time, which is the term of interest. The term of interest, aortic PWV x time, captures change over time in the outcome attributed to aortic PWV reflecting either gray matter atrophy or WMH growth. Random effects included the intercept and time by each individual participant. Models were repeated testing an aortic PWV x APOE-ε4 status x time interaction term and an aortic PWV x diagnosis x time interaction term, followed by stratification by APOE-ε4 status (carrier or noncarrier) and by diagnosis (NC or MCI). Significance was set a priori at p<0.05. False discovery rate correction for multiple comparisons was performed using the Benjamini-Hochberg procedure by MRI sequence adjusting for 7 gray matter volume analyses and separately adjusting for 5 WMH volume analyses. False discovery rate correction was used for all hypothesis-testing models but not for descriptive comparisons across groups. To assess the role blood pressure plays in associations between arterial stiffness and structural brain changes, we performed 2 additional sets of post hoc analyses. First, we repeated all main effect models with systolic blood pressure as the predictor instead of aortic PWV. Second, we repeated main effect models with systolic blood pressure as an independent covariate instead of systolic blood pressure as a component of the FSRP. Statistical analyses were performed in R 3.5.2 (www.r-project.org).
3. Results
3.1. Participants Characteristics
Participants included 278 adults (73±7 years, 58% male, 87% self-identified as non-Hispanic White), including 159 with NC and 119 with MCI, from the Vanderbilt Memory & Aging Project (n=335). Two hundred thirty-four participants were included at 18 months, 213 at 3 years, 181 at 5 years, and 54 at 7 years. During follow-up, 14 NC participants (8%) converted to MCI and 30 MCI participants (25%) converted to dementia based on the Clinical Dementia Rating global score. No participants were excluded based on a change in cognitive diagnosis across the follow-up period, but participants who dropped out of the study over the follow-up tended to have worse health at baseline. Aortic PWV ranged from 3.5 to 25.5 (8.2±3.1) m/s and did not differ between NC and MCI participants (p=0.93). Participants with at-least 1 follow-up visit (n=240) were followed for an average of 4.9±1.6 years up to 7 years with PWV measurements occurring from September 2012 to November 2014 and longitudinal brain MRI measurements occurring from September 2012 to June 2021. Mean annual volume changes were −5.10 cm3 for total gray matter and 1.95 cm3 for total WMHs. See Table 1 for more details. To assess possible regression to the mean effects on annual change in brain MRI measurements, we performed post hoc correlation analyses between each set of epochs for all longitudinal outcomes. Correlation coefficients ranged from 0.72 to 0.98 and were ≥0.88 for outcomes with significant main effect associations (ie, hippocampus volume, occipital lobe gray matter volume, and temporal lobe WMH volume), indicating any regression to the mean effect is small.
Table 1.
Participant Characteristics
| Total n=278 |
NC n=159 |
MCI n=119 |
p-value | |
|---|---|---|---|---|
| Baseline Characteristics | ||||
|
| ||||
| Age, y | 73±7 | 72±7 | 73±7 | 0.39 |
| Sex, % male | 58 | 58 | 58 | 0.93 |
| Race, % non-Hispanic white | 87 | 87 | 87 | 0.95 |
| Education, y | 16±3 | 16±3 | 15±3 | <0.001* |
| APOE-ε4, % carrier | 35 | 29 | 43 | 0.02* |
| Framingham Stroke Risk Profile, total† | 12.3±4.3 | 11.9±4.3 | 12.8±4.2 | 0.06 |
| Systolic blood pressure, mmHg | 142±18 | 140±17 | 144±18 | 0.02* |
| Antihypertensive medication usage, % | 53 | 53 | 52 | 0.90 |
| Diabetes, % | 18 | 16 | 20 | 0.41 |
| Cigarette smoking, % current | 2 | 1 | 3 | 0.23 |
| Prevalent CVD, % | 5 | 6 | 3 | 0.37 |
| Atrial fibrillation, % | 6 | 6 | 7 | 0.71 |
| Left ventricular hypertrophy, % | 4 | 3 | 6 | 0.27 |
| Intracranial volume, cm3 | 1507±154 | 1503±154 | 1512±155 | 0.78 |
| Pulse wave velocity, m/s | 8.2±3.1 | 8.1±3.0 | 8.3±3.3 | 0.93 |
|
Gray Matter Neuroimaging Markers, mm 3 /year | ||||
| Total gray matter annual change | −5101±8621 | −4741±8590 | −5620±8684 | 0.29 |
| Frontal gray matter annual change | −2159±4261 | −2143±4424 | −2181±4036 | >0.99 |
| Temporal gray matter annual change | −691±1494 | −458±1376 | −1027±1596 | 0.005* |
| Parietal gray matter annual change | −1336±2097 | −1319±2140 | −1362±2044 | 0.42 |
| Occipital gray matter annual change | −447±1054 | −455±956 | −435±1186 | 0.79 |
| Hippocampal annual change | −77±96 | −62±76 | −98±116 | 0.01* |
| Inferior lateral ventricle annual change | 157±204 | 100±108 | 239±272 | <0.001* |
|
WMH Neuroimaging Markers, mm 3 /year | ||||
| Total WMH annual change | 1954±2953 | 1365±1806 | 2793±3923 | 0.001* |
| Frontal WMH annual change | 1110±1525 | 825±986 | 1516±2000 | 0.009* |
| Temporal WMH annual change | 103±347 | 66±310 | 154±390 | 0.002* |
| Parietal WMH annual change | 541±864 | 381±563 | 769±1131 | 0.02* |
| Occipital WMH annual change | 201±663 | 93±395 | 354±900 | 0.01* |
Note. APOE-ε4 indicates apolipoprotein E ε4 allele; CVD, cardiovascular disease; MCI, mild cognitive impairment; NC, normal cognition.
Meets the a priori significance threshold of 0.05
A modified Framingham Stroke Risk Profile Score was included in statistical models, which excluded points assigned to age (total=6.5±3.1, NC=6.2±3.0, MCI=6.8±3.2). Annual change values represent the average change in tissue volume per year for each region of interest.
3.2. Aortic PWV and Baseline Gray Matter and WMH Volumes
Baseline aortic PWV was not cross-sectionally related to gray matter (p>0.06) or WMH volumes (p>0.48). The aortic PWV x APOE-ε4 status interaction term was unrelated to both cross-sectional gray matter and WMH volumes (p>0.28). The aortic PWV x cognitive diagnosis interaction term was significantly associated with cross-sectional occipital lobe gray matter volume (p=0.03), but all remaining associations with cross-sectional gray matter and WMH volumes were null (p>0.14).
3.3. Aortic PWV and Longitudinal Gray Matter Volume Changes
Higher baseline aortic PWV was associated with greater decrease in gray matter volume over the follow-up period in the hippocampus (β=−3.6 [mm3/year]/[m/s], 95% CI=−7.2 to −0.02, p=0.049) and occipital lobe (β=−34.2 [mm3/year]/[m/s], 95% CI=−67.8 to −0.55, p=0.046). See Table 2 for details, including false discovery rate-corrected P values, and Figure 2 for illustration. All remaining P values throughout the article are uncorrected. In sensitivity analyses excluding participants with CVD and atrial fibrillation, the association between aortic PWV and greater decrease in gray matter volume in the hippocampus (β=−4.2 [mm3/year]/[m/s], 95% CI=−8.3 to −0.11, p=0.04) persisted, but the association with occipital lobe volume was attenuated. When excluding outliers, associations between baseline aortic PWV and longitudinal hippocampal and occipital lobe gray matter volumes were attenuated. See (Table I in the Data Supplement). We performed post hoc analyses where we log-transformed our PWV values to account for any skewness. Results from these analyses were largely similar to our primary analyses with all previously significant models remaining statistically significant. When models were repeated using systolic blood pressure as a continuous variable covariate (rather than as a component of the FSRP), the association between aortic PWV and longitudinal occipital lobe gray matter volume was attenuated (p=0.06). See (Table II in the Data Supplement).
Table 2.
Aortic PWV Associations with Longitudinal Gray Matter Volume and WMH Volume
| Gray Matter Neuroimaging Markers
((mm3/year)/(m/s)) | ||||
|---|---|---|---|---|
| β | 95% CI | p-value | FDR p-value | |
| Total gray matter | −184.8 | −510.9, 141.4 | 0.27 | 0.49 |
| Frontal gray matter | −21.94 | −189.6, 145.7 | 0.80 | 0.92 |
| Temporal gray matter | −42.52 | −96.75, 11.71 | 0.12 | 0.40 |
| Parietal gray matter | −51.40 | −129.7, 26.89 | 0.20 | 0.43 |
| Occipital gray matter | −34.19 | −67.83, −0.55 | 0.046* | 0.21 |
| Hippocampus | −3.61 | −7.19, −0.02 | 0.049* | 0.21 |
| Inferior lateral ventricle | 5.76 | −2.26, 13.77 | 0.16 | 0.41 |
| WMH Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total WMH | 30.82 | −62.37, 124.0 | 0.52 | 0.83 |
| Frontal WMH | −2.74 | −53.15, 47.68 | 0.92 | 0.92 |
| Temporal WMH | 17.04 | 7.17, 26.92 | <0.001* | 0.009 |
| Parietal WMH | 8.69 | −21.68, 39.05 | 0.57 | 0.83 |
| Occipital WMH | 0.81 | −15.95, 17.57 | 0.92 | 0.92 |
Note. Analyses performed on n=278 participants. Models were adjusted for age, sex, race/ethnicity, education, APOE-ε4 status, cognitive diagnosis, and Framingham stroke risk profile (excluding points assigned for age). The parameter estimates (β) are for the PWV×time interaction term and is interpreted as the annual changes of outcomes associated with 1 unit change in PWV (per 1 m/s). FDR indicates false discovery rate; PWV, pulse wave velocity; and WMH, white matter hyperintensity.
Meets the a priori significance threshold of 0.05
Figure 2. PWV and Longitudinal Cerebral Structural Changes.
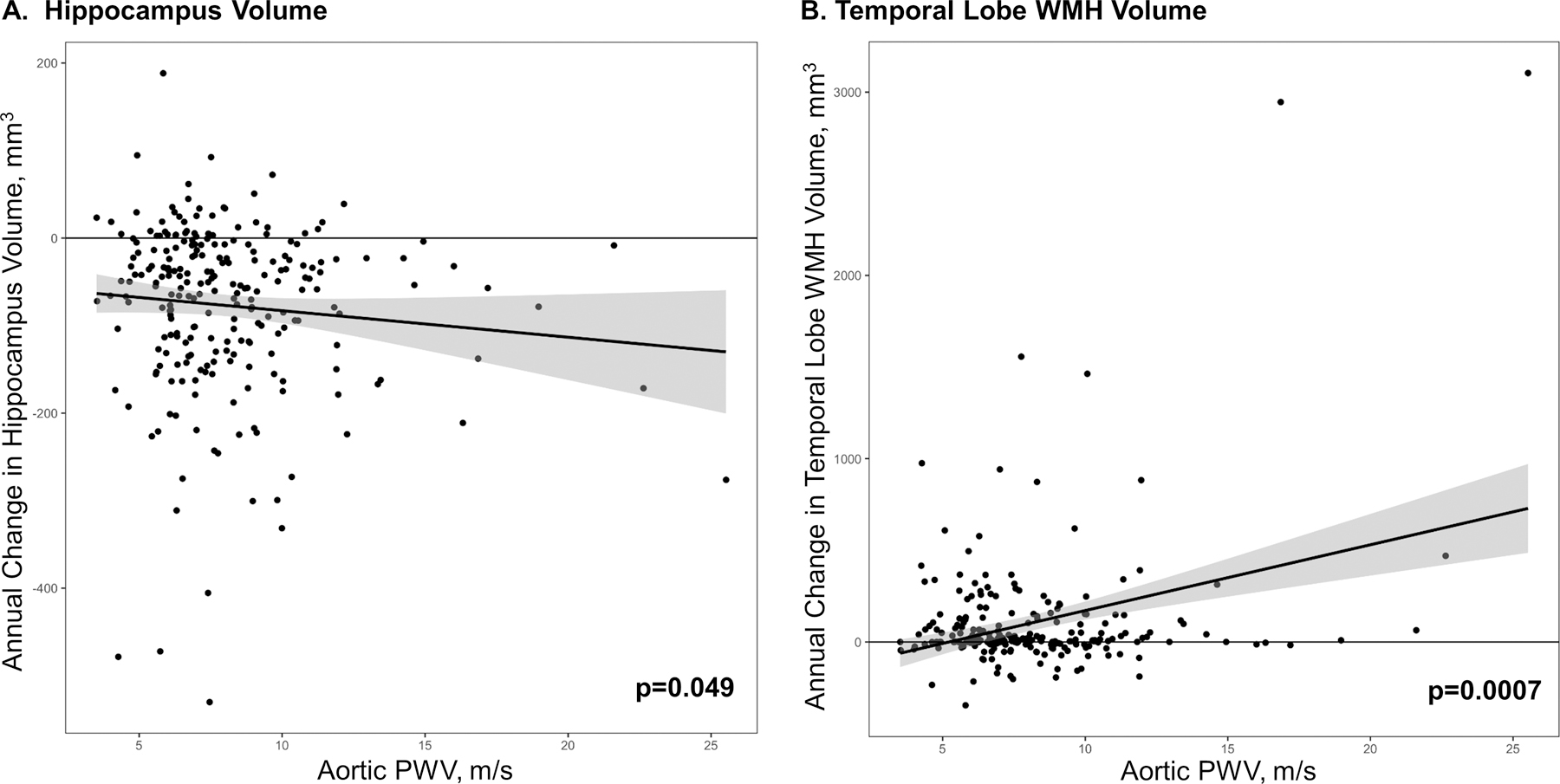
Baseline PWV associations with a) Hippocampus Volume and b) Temporal Lobe WMH Volume. PWV, pulse wave velocity. WMH, white matter hyperintensity. When outliers are excluded, associations with hippocampus volume and temporal WMH volume are attenuated (p>0.14).
The aortic PWV x APOE-ε4 status x time interaction term was unrelated to any longitudinal gray matter volume changes (P>0.11; Table 3).
Table 3.
Aortic PWV x APOE-ε4 x Time Interactions and Stratifications by APOE-ε4 Status with Longitudinal Magnetic Resonance Imaging Outcomes
| β | 95 % CI | p-value | FDR p-value | |
|---|---|---|---|---|
|
APOE-ε4 Status Interaction Gray
Matter Neuroimaging Markers
((mm
3
/year)/(m/s))
| ||||
| Total gray matter | −164.8 | −903.8, 574.2 | 0.66 | 0.86 |
| Frontal gray matter | −14.69 | −384.8, 355.4 | 0.94 | 0.94 |
| Temporal gray matter | −99.42 | −232.9, 34.02 | 0.14 | 0.47 |
| Parietal gray matter | −19.15 | −187.7, 149.4 | 0.82 | 0.94 |
| Occipital gray matter | −29.49 | −111, 51.97 | 0.48 | 0.69 |
| Hippocampus | −4.45 | −13.55, 4.66 | 0.34 | 0.55 |
| Inferior lateral ventricle | 13.32 | −2.84, 29.47 | 0.11 | 0.46 |
| APOE-ε4 Status Interaction WMH Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total WMH | −138.9 | −355.8, 78.08 | 0.21 | 0.54 |
| Frontal WMH | −61.02 | −180.7, 58.65 | 0.32 | 0.55 |
| Temporal WMH | −27.16 | −50.30, −4.02 | 0.02* | 0.14 |
| Parietal WMH | −40.71 | −111.9, 30.53 | 0.26 | 0.55 |
| Occipital WMH | 2.50 | −38.78, 43.78 | 0.91 | 0.94 |
|
APOE-ε4 Carriers Gray Matter Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total gray matter | −485.4 | −1190, 219.6 | 0.18 | 0.37 |
| Frontal gray matter | −106.6 | −448.2, 234.9 | 0.54 | 0.69 |
| Temporal gray matter | −132.1 | −268.4, 4.21 | 0.06 | 0.26 |
| Parietal gray matter | −100.1 | −252.8, 52.52 | 0.20 | 0.37 |
| Occipital gray matter | −69.59 | −152.9, 13.74 | 0.10 | 0.30 |
| Hippocampus | −7.97 | −17.96, 2.01 | 0.12 | 0.30 |
| Inferior lateral ventricle | 21.50 | −0.92, 43.92 | 0.06 | 0.26 |
| APOE-ε4 Carriers WMH Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total WMH | −58.09 | −320.3, 204.1 | 0.66 | 0.69 |
| Frontal WMH | −35.55 | −172.9, 101.8 | 0.61 | 0.69 |
| Temporal WMH | −6.98 | −23.84, 9.87 | 0.42 | 0.67 |
| Parietal WMH | −19.05 | −105.3, 67.19 | 0.66 | 0.69 |
| Occipital WMH | 8.11 | −31.63, 47.85 | 0.69 | 0.69 |
|
APOE-ε4 Non-carriers Gray Matter Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total gray matter | −89.79 | −465.9, 286.4 | 0.64 | 0.82 |
| Frontal gray matter | 15.46 | −183.9, 214.8 | 0.88 | 0.88 |
| Temporal gray matter | −28.64 | −84.82, 27.54 | 0.32 | 0.51 |
| Parietal gray matter | −30.13 | −123.3, 63.09 | 0.53 | 0.76 |
| Occipital gray matter | −25.96 | −62.2, 10.29 | 0.16 | 0.35 |
| Hippocampus | −3.48 | −6.98, 0.02 | 0.05 | 0.33 |
| Inferior lateral ventricle | 4.88 | −2.07, 11.83 | 0.17 | 0.35 |
| APOE-ε4 Non-carriers WMH Neuroimaging Markers ((mm 3 /year)/(m/s)) | ||||
| Total WMH | 70.26 | −20.74, 161.3 | 0.13 | 0.35 |
| Frontal WMH | 10.10 | −40.96, 61.17 | 0.70 | 0.82 |
| Temporal WMH | 23.95 | 11.59, 36.32 | <0.001* | 0.003 |
| Parietal WMH | 19.73 | −9.78, 49.23 | 0.19 | 0.35 |
| Occipital WMH | 2.08 | −16.20, 20.37 | 0.82 | 0.88 |
Note. Analyses performed on n=278 participants and subsequently stratified by APOE-ε4 status for n=97 APOE-ε4 positive participants and n=181 APOE-ε4 negative participants. The interaction term was aortic PWV x time x APOE-ε4 status. Models were adjusted for age, sex, race/ethnicity, education, APOE-ε4 status, and Framingham Stroke Risk Profile (excluding points assigned for age). APOE-ε4, apolipoprotein E ε4 allele. CI, confidence interval; FDR p-value, false discovery rate corrected p-value. For the APOE-ε4 Status interaction model, the parameter estimates (β) are for the PWV x APOE-ε4 x time interaction term and is interpreted as the difference in annual changes of outcomes between APOE-ε4 positive participants and APOE-ε4 negative participants associated with one unit change in PWV (per 1 m/s). For stratified models by APOE-ε4 status, the parameter estimates (β) are for the PWV x time interaction term and is interpreted as the annual changes of outcomes associated with one unit change in PWV (per 1 m/s).
Meets the a priori significance threshold of 0.05
The aortic PWV x diagnosis x time interaction term was unrelated to any longitudinal gray matter changes (P>0.32; Table III in the Data Supplement).
3.4. Baseline Aortic PWV and Longitudinal WMH Volume Changes
Higher baseline aortic PWV was associated with greater increase in WMH volume in the temporal lobe (β=17.0 [mm3/year]/[m/s], 95% CI=7.2 to 26.9, p<0.001). When excluding participants with CVD and atrial fibrillation, results were similar. In sensitivity analyses excluding outliers, associations between baseline aortic PWV and longitudinal temporal lobe WMH volume was attenuated (p=0.40). We performed post hoc analyses where we log-transformed our PWV values to account for any existing skewness. The results from these analyses were largely similar with all previously significant models remaining statistically significant. When models were repeated using systolic blood pressure as a continuous variable covariate (rather than as a component of the FSRP), results were unchanged.
The aortic PWV x APOE-ε4 x time interaction term was related to temporal lobe WMH volume (β=−27.2 [mm3/year]/[m/s], 95% CI=−50.3 to −4.0, p=0.02). Results persisted when excluding participants with CVD and atrial fibrillation, but when excluding outliers, results were attenuated (p=0.15). In stratified models, results were null among APOE-ε4 carriers (P>0.42). Among APOE-ε4 non-carriers, higher baseline aortic PWV was associated with greater increase in WMH volume in the temporal lobe (β=24.0 [mm3/year]/[m/s], 95% CI=11.6 to 36.3, P<0.001; Table 3). Results persisted after excluding for CVD and atrial fibrillation but were attenuated when excluding outliers (p=0.07).
The aortic PWV x diagnosis x time interaction term was unrelated to longitudinal WMHs (P>0.21; Table III in the Data Supplement).
3.5. Systolic Blood Pressure and Longitudinal Brain Changes
Systolic blood pressure was unrelated to any longitudinal gray matter volume changes (p>0.16). Systolic blood pressure was significantly associated with greater increase in WMH volume in the temporal lobe (β=1.78 [mm3/y]/[m/s]; [95% CI, 0.12–3.43] P=0.04; Table IV in the Data Supplement).
4. Discussion
This study leveraged multimodal MRI techniques to quantify aortic PWV and structural brain changes among community-dwelling older adults to better understand detailed associations between central arterial stiffness and longitudinal brain structure. All cross-sectional results assessing associations between aortic PWV and gray matter volumes, as well as WMH volumes were null. Higher baseline aortic PWV was associated with greater decrease in gray matter volume over the mean 4.2-year follow-up period in the hippocampus and occipital lobe. Neither APOE-ε4 nor cognitive diagnosis modified associations. Higher baseline aortic PWV—a measure of the degree of aortic stiffness—was associated with greater increase in WMH volumes in the temporal lobe over the follow-up period. While associations were not modified by diagnosis, the baseline aortic PWV x APOE-ε4 interaction term was related to longitudinal WMH volume in the temporal lobe. Associations between higher baseline aortic PWV and greater increase in WMH volume appear to be driven by APOE-ε4 noncarriers.
Our results suggest that central arterial stiffening predicts greater decrease in gray matter volume in the hippocampus. These observations are particularly interesting considering the regions affected by aortic stiffening overlap with regions that accumulate Alzheimer’s disease pathology early in the disease process. Indeed, higher aortic stiffness has been related to increased cerebral amyloid deposition in older adults.37,38 Importantly though, baseline models relating PWV to gray matter and WMH volumes in this cohort were null, suggesting that aortic stiffening contributes little to interindividual differences at baseline. PWV does, however, predict the rate of neurodegeneration, suggesting aortic stiffening may create susceptibility to more rapid change once a pathological process is initiated. These observations provide subtle yet potentially important insights about drivers and rate of neurodegeneration that warrant further inquiry.
We previously reported higher aortic PWV was cross-sectionally associated with lower cerebral blood flow in several brain regions, including the temporal lobe, which contains the hippocampus, and occipital lobe in subsets of the cohort.29 Reduced cerebral blood flow due to higher PWV is likely the result of microvascular remodeling and higher vascular resistance.29 Chronic reductions in cerebral blood flow may lead to unmet cerebral metabolic demands, which disrupt cerebral homeostasis and result in cell dysfunction, death, and subsequent atrophy due to excitotoxicity.39,40 Interestingly, the hippocampus and temporal lobes are regions with particularly high energy consumption. The hippocampus is one of the most densely populated brain regions in terms of synapses,41,42 and the medial temporal lobes are involved in the highly active default mode network.43 The metabolic demand of these regions may make them particularly vulnerable to excitotoxicity. However, it is plausible that other damaging cascades, such as oxidative stress or blood-brain barrier breakdown, play a role in the longitudinal atrophy observed here.
Our second major observation is that central arterial stiffening contributes to greater increase in WMH volume in the temporal lobe. One explanation aligned with the aforementioned discussion is that arterial stiffness is linked to reduced cerebral blood flow,29 resulting in oligemia and possibly ischemia. Ischemia is a well-established driver of WMH formation in animal models,44 and in vivo ischemia is related to WMHs in humans.45 Alternatively, blood-brain barrier breakdown—a purported consequence of increased pulsatility46—results in pro-inflammatory protein infiltration into the parenchyma47–50 creating a toxic environment that can result in demyelination51,52 and axonal loss.47 As noted above, the temporal53 lobe is metabolically demanding. Brain regions with increased neuronal activity could be preferentially susceptible to white matter damage in the context of arterial stiffening since damaged vessels would impede adequate nutrient delivery. One additional possibility is that WMH can appear over time secondary to neurodegeneration. The regional overlap between WMH formation and gray matter atrophy in the temporal lobe seen here may just be a consequence of neurodegeneration. When comparing temporal lobe models, we see that the effect size for gray matter atrophy is about 2 to 3× as large as the effect size for WMH growth suggesting greater damage in the gray matter. Given that both effect sizes are similar magnitude, we cannot rule out that neurodegeneration played a role in the longitudinal formation of WMHs.
Baseline aortic PWV interacted with APOE-ε4 carrier status on greater increase in WMH volume in the temporal lobe. Higher PWV was associated with greater increase in WMH volume in the temporal lobe among noncarriers rather than carriers. These results are counter to expectation as APOE-ε4 is cross-sectionally associated with more WMHs54 and worse white matter integrity on diffusion tensor imaging.55 It is possible that the detrimental effect of APOE-ε4 on white matter leads to accumulating damage over the lifespan that obscures any effect PWV may have on longitudinal WMHs later in life. APOE transports cholesterol,56 a primary component of myelin,57 but APOE-ε4 is less efficient than other allele forms at cholesterol transport, leading to less myelination of axons. Additionally, APOE-ε4 carriers have greater white matter damage visible on multiple neuroimaging sequences,58 even as early as infancy.59 Additionally, when myelin breaks down during normal aging, APOE helps transport cholesterol away from the site of damage to prepare for remyelination,60 but due to inefficiencies with APOE-ε4, less remyelination takes place in APOE-ε4 carriers in older age.61 Thus, while arterial stiffness may affect WMHs among older APOE-ε4 carriers, it may be masked by the overwhelming effect that APOE-ε4 has on white matter damage and myelination over the lifespan. An additional possibility is that regions with WMHs among APOE-ε4 carriers have greater cell death and atrophy, resulting in an apparent stagnation or shrinkage of WMH volume.62 Finally, it should be noted that the interaction with APOE-ε4 is attenuated when removing outliers, so these results may be the result of a type I error and highlight the need for replication.
All post hoc main effect models relating systolic blood pressure to longitudinal brain changes were null except for greater longitudinal increase in temporal lobe WMHs. Aortic PWV may be more predictive of longitudinal structural brain changes compared with systolic blood pressure. These findings point to aortic PWV as a strong predictor of future structural brain changes, which may be because aortic PWV is sensitive to early arterial stiffening changes before systolic blood pressure increases.2,63
The current study has many strengths, including detailed multimodel MRI analysis, a direct noninvasive measure of blood flow in the aorta on cardiac MR to capture PWV, and thorough covariate acquisition. While the present study has many strengths, some limitations are worth mentioning when interpreting our results. Given the college-educated, predominantly White, older sample, generalizability of findings is limited. The possibility exists that in a less healthy cohort with increased aortic stiffness and more CVD, the present observations would be more robust (which is, in part, supported by our sensitivity analyses for which findings were attenuated when excluding outliers). Attrition occurred among the less healthy participants, which likely biases our results to the null hypothesis.
Focusing on how age-related systemic cardiovascular changes influence neurodegeneration is an essential aspect of understanding the multifactorial contributors to adverse cognitive aging and dementia risk. The present research serves to increase our knowledge by showing aortic stiffness is associated with longitudinal gray matter and white matter damage, particularly in the temporal lobes.
Supplementary Material
Highlights.
Higher baseline aortic pulse wave velocity was significantly associated with greater decrease in hippocampal and occipital lobe gray matter volumes.
Higher baseline aortic pulse wave velocity was significantly associated with greater increase in temporal lobe white matter hyperintensity volumes.
Arterial stiffening may preferentially affect certain highly active brain regions like the temporal lobes. These same regions are affected early in the course of Alzheimer’s disease.
Acknowledgements
We would like to thank the dedicated Vanderbilt Memory & Aging Project participants, their loved ones, and our devoted staff and trainees who contributed to recruitment, screening, enrollment, and follow-up of the cohort. Statistical analysis was conducted by O.A. Khan and D. Liu.
Sources of Funding
This research was supported by F31-AG066358 (CWB), T32-AG058524 (CWB), F30-AG064847 (EEM), T-32 GM007347 (EEM), F31-AG059345 (FEC), Howard Hughes Medical Institute James H. Gilliam Fellowship for Advanced Study (FEC), Alzheimer’s Association IIRG-08-88733 (ALJ), R01-AG034962 (ALJ), R01-NS100980 (ALJ), K24-AG046373 (ALJ), Paul B. Beeson Career Development Award in Aging K23-AG045966 (KAG), K01-AG049164 (TJH), UL1-TR000445 and UL1-TR002243 (Vanderbilt Clinical Translational Science Award), S10-OD023680 (Vanderbilt’s High-Performance Computer Cluster for Biomedical Research), P20-AG068082 (Vanderbilt Alzheimer’s Disease Research Center), and the Vanderbilt Memory & Alzheimer’s Center.
Non Standard Abbreviations
- APOE-ε4
Apolipoprotein E ε4
- CVD
Cardiovascular disease
- FSRP
Framingham stroke risk profile
- MCI
Mild cognitive impairment
- NC
Normal Cognition
- PWV
Pulse wave velocity
- WMH
White matter hyperintensity
- MRI
magnetic resonance imaging
Footnotes
Statistical Analysis conducted by Omair A. Khan, MAS and Dandan Liu, PhD, Department of Biostatistics, Vanderbilt University Medical Center
Disclosures:
Corey Bown reports no disclosures.
Omair Khan reports no disclosures.
Elizabeth Moore reports no disclosures.
Dr. Liu reports no disclosures.
Dr. Pechman reports no disclosures.
Francis Cambronero reports no disclosures.
James Terry reports no disclosures.
Dr. Nair reports no disclosures.
Dr. Davis reports no disclosures.
Dr. Gifford reports no disclosures.
Dr. Landman reports no disclosures.
Dr. Hohman reports no disclosures.
Dr. Carr reports no disclosures.
Dr. Jefferson reports no disclosures.
References
- 1.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. American journal of physiology Heart and circulatory physiology 2016;310:H365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 2014;64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Manipulation of ascending aortic pressure and flow wave reflections with the Valsalva maneuver: relationship to input impedance. Circulation 1981;63:122–132. [DOI] [PubMed] [Google Scholar]
- 5.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008;51:99–104. [DOI] [PubMed] [Google Scholar]
- 6.Zeki Al Hazzouri A, Newman AB, Simonsick E, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke 2013;44:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui C, Sekikawa A, Kuller LH, et al. Aortic Stiffness is Associated with Increased Risk of Incident Dementia in Older Adults. J Alzheimers Dis 2018;66:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Od Montgolfier, Pinçon A, Pouliot P, et al. High Systolic Blood Pressure Induces Cerebral Microvascular Endothelial Dysfunction, Neurovascular Unit Damage, and Cognitive Decline in Mice. Hypertension 2019;73:217–228. [DOI] [PubMed] [Google Scholar]
- 9.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke 2007;38:674–676. [DOI] [PubMed] [Google Scholar]
- 10.Berliocchi L, Bano D, Nicotera P. Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci 2005;360:2255–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature reviews Molecular cell biology 2003;4:552–565. [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C The pathobiology of vascular dementia. Neuron 2013;80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nature medicine 2013;19:1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011;42:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Wang D, Lan L, Fan Y. Multiple Factors Involved in the Pathogenesis of White Matter Lesions 2017;2017:9372050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trauninger A, Leel-Ossy E, Kamson DO, et al. Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. The journal of headache and pain 2011;12:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai FF, Ye YC, Chen SY, et al. Arterial Stiffness and Cerebral Small Vessel Disease. Frontiers in Neurology 2018;9:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CY, Cheng HM, Chen SP, et al. White matter hyperintensities in migraine: Clinical significance and central pulsatile hemodynamic correlates. Cephalalgia : an international journal of headache 2018;38:1225–1236. [DOI] [PubMed] [Google Scholar]
- 19.Maillard P, Mitchell GF, Himali JJ, et al. Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity: A Continuum of Injury. Stroke 2017;48:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tap L, van Opbroek A, Niessen WJ, Smits M, Mattace-Raso FU. Aortic stiffness and brain integrity in elderly patients with cognitive and functional complaints. Clin Interv Aging 2018;13:2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King KS, Chen KX, Hulsey KM, et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology 2013;267:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katulska K, Wykretowicz M, Minczykowski A, et al. Gray matter volume in relation to cardio-vascular stiffness. Journal of the neurological sciences 2014;343:100–104. [DOI] [PubMed] [Google Scholar]
- 23.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012;485:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suri S, Mackay CE, Kelly ME, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE epsilon4 allele. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2015;11:648–657.e641. [DOI] [PubMed] [Google Scholar]
- 25.Jefferson AL, Gifford KA, Acosta LM, et al. The Vanderbilt Memory & Aging Project: Study design and baseline cohort overview. J Alzheimers Dis 2016;52:539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 27.Aisen PS, Petersen RC, Donohue MC, et al. Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson AL, Cambronero FE, Liu D, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018;138:1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentland AL, Grist TM, Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovascular diagnosis and therapy 2014;4:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorniak K, Heiberg E, Hellmann M, et al. Required temporal resolution for accurate thoracic aortic pulse wave velocity measurements by phase-contrast magnetic resonance imaging and comparison with clinical standard applanation tonometry. BMC cardiovascular disorders 2016;16:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asman AJ, Landman BA. Formulating spatially varying performance in the statistical fusion framework. IEEE transactions on medical imaging 2012;31:1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012;59:3774–3783. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke 1994;25:40–43. [DOI] [PubMed] [Google Scholar]
- 35.Apostolova LG, Green AE, Babakchanian S, et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer disease and associated disorders 2012;26:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartos A, Gregus D, Ibrahim I, Tintěra J. Brain volumes and their ratios in Alzheimeŕs disease on magnetic resonance imaging segmented using Freesurfer 6.0. Psychiatry research Neuroimaging 2019;287:70–74. [DOI] [PubMed] [Google Scholar]
- 37.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology 2013;81:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial Stiffness and beta-Amyloid Progression in Nondemented Elderly Adults. JAMA Neurol 2014;71:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences 1999;22:391–397. [DOI] [PubMed] [Google Scholar]
- 40.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell calcium 2010;47:122–129. [DOI] [PubMed] [Google Scholar]
- 41.Jinno S, Kosaka T. Stereological estimation of numerical densities of glutamatergic principal neurons in the mouse hippocampus. Hippocampus 2010;20:829–840. [DOI] [PubMed] [Google Scholar]
- 42.Keller D, Ero C, Markram H. Cell Densities in the Mouse Brain: A Systematic Review. Frontiers in neuroanatomy 2018;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grajski KA, Bressler SL. Differential medial temporal lobe and default-mode network functional connectivity and morphometric changes in Alzheimer’s disease. NeuroImage Clinical 2019;23:101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol 1994;87:484–492. [DOI] [PubMed] [Google Scholar]
- 45.Yao H, Sadoshima S, Ibayashi S, Kuwabara Y, Ichiya Y, Fujishima M. Leukoaraiosis and dementia in hypertensive patients. Stroke 1992;23:1673–1677. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Polite F, Martorell J, Del Rey-Puech P, et al. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J Cereb Blood Flow Metab 2017;37:2614–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz GG, Pacheco-Moises FP, Macias-Islas MA, et al. Role of the blood-brain barrier in multiple sclerosis. Archives of medical research 2014;45:687–697. [DOI] [PubMed] [Google Scholar]
- 48.Becher B, Dodelet V, Fedorowicz V, Antel JP. Soluble tumor necrosis factor receptor inhibits interleukin 12 production by stimulated human adult microglial cells in vitro. J Clin Invest 1996;98:1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. Journal of clinical immunology 2008;28:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science (New York, NY) 2007;318:1141–1143. [DOI] [PubMed] [Google Scholar]
- 51.Ljubisavljevic S, Stojanovic I. Neuroinflammation and demyelination from the point of nitrosative stress as a new target for neuroprotection. Reviews in the neurosciences 2015;26:49–73. [DOI] [PubMed] [Google Scholar]
- 52.Ljubisavljevic S Oxidative Stress and Neurobiology of Demyelination. Molecular neurobiology 2016;53:744–758. [DOI] [PubMed] [Google Scholar]
- 53.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995;16:271–278; discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 54.Lyall DM, Cox SR, Lyall LM, et al. Association between APOE e4 and white matter hyperintensity volume, but not total brain volume or white matter integrity. Brain imaging and behavior 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persson J, Lind J, Larsson A, et al. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology 2006;66:1029–1033. [DOI] [PubMed] [Google Scholar]
- 56.Poirier J Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging 2005;26:355–361. [DOI] [PubMed] [Google Scholar]
- 57.Saher G, Brugger B, Lappe-Siefke C, et al. High cholesterol level is essential for myelin membrane growth. Nature neuroscience 2005;8:468–475. [DOI] [PubMed] [Google Scholar]
- 58.Operto G, Cacciaglia R, Grau-Rivera O, et al. White matter microstructure is altered in cognitively normal middle-aged APOE-ε4 homozygotes. Alzheimer’s research & therapy 2018;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dean DC 3rd, Jerskey BA, Chen K, et al. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-sectional Imaging Study. JAMA Neurol 2014;71:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantuti-Castelvetri L, Fitzner D. Defective cholesterol clearance limits remyelination in the aged central nervous system 2018;359:684–688. [DOI] [PubMed] [Google Scholar]
- 61.Bartzokis G, Lu PH, Geschwind DH, et al. Apolipoprotein E affects both myelin breakdown and cognition: implications for age-related trajectories of decline into dementia. Biological psychiatry 2007;62:1380–1387. [DOI] [PubMed] [Google Scholar]
- 62.Dwyer MG, Bergsland N. Atrophied Brain Lesion Volume: A New Imaging Biomarker in Multiple Sclerosis 2018;28:490–495. [DOI] [PubMed] [Google Scholar]
- 63.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension 2005;45:426–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to participant consent restrictions in data sharing, a subset of data is available for purposes of reproducing results or replicating procedures. The data, analytic methods, and study materials can be obtained by contacting the corresponding author. The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. Written informed consent was obtained before data collection.


