Figure 2: Experimental insight into the mechanism of AC/DC excipient stabilization.
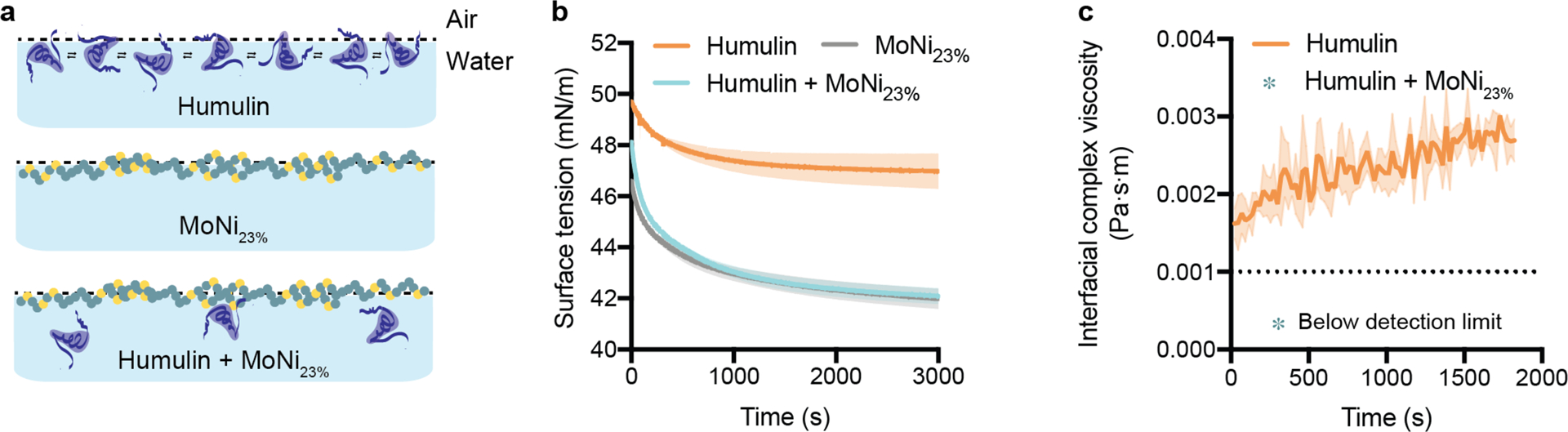
a, Illustration of proposed stabilization mechanism: (i) In commercial Humulin, monomers at the interface have associative interactions. (ii) Alone, MoNi23% occupies the interface without the presence of insulin. (iii) In combination with Humulin formulations, MoNi23% disrupts insulin-insulin surface interactions, providing a mechanism for inhibiting aggregation. b, Surface tension measurements of Humulin, MoNi23% (0.01 wt.%) formulated with formulation excipients, and Humulin formulated with MoNi23% (0.01 wt.%) (n=2). c, Interfacial rheology measurements of Humulin. Measurements for Humulin formulated with MoNi23% (0.01 wt.%) fell below the resolution of the instrument, indicating that there is no protein aggregation at the interface (n=3).
