Abstract
Immune-related adverse events, including autoimmune toxicity, may develop as a consequence of immune-checkpoint inhibitor (ICI) cancer therapy. Cytokine release syndrome (CRS) is a severe and life-threatening cytokine-associated toxicity that can develop after adoptive T-cell therapy. We herein report a rare case of severe CRS after ICI therapy for advanced non-small-cell lung cancer. He presented with a prolonged high fever, cardiogenic shock, and disseminated intravascular coagulation after the first course of programed death ligand-1 inhibitor and platinum-based doublet chemotherapy. He recovered by steroid pulse therapy and tocilizumab. CRS is a rare but life-threatening adverse event of ICI therapy and therefore warrants awareness.
Keywords: immune-checkpoint therapy, cytokine release syndrome, tocilizumab
Introduction
Immune-checkpoint inhibitors (ICIs) have become a mainstay in cancer treatment. Situations where autoimmune toxicity develops in the context of immune-checkpoint therapy are referred to as immune-related adverse events (irAEs) (1). Various irAEs have been reported, and moderate-to-severe cases can be managed by the administration of corticosteroids or other immunosuppressive agents.
Cytokine release syndrome (CRS) is a cytokine-associated toxicity observed after administration of chimeric antigen receptor T-cell (CAR-T) therapy (2). CRS can be severe and life-threatening. The anti-IL-6 receptor antibody tocilizumab is an immunosuppressive agent that has shown efficacy for severe CRS (3).
Very rarely, CRS has been reported as a side effect of ICI. We herein report a case of CRS after platinum-based doublet chemotherapy combined with ICI therapy successfully treated by corticosteroids and tocilizumab.
Case Report
A 53-year-old man presented to our hospital with back pain and an abnormal shadow in the upper area of the right lung. He was diagnosed with stage IVb lung adenocarcinoma (cT3N3M1c) with distant metastasis to the left adrenal gland and multiple bones.
He had a history of controlled type 2 diabetes and hyperlipidemia, and his Eastern Cooperative Oncology Group performance status was 1. He received radiation therapy (30 Gray) to the 10th thoracic spine before the administration of systemic chemotherapy. Twelve days after he completed radiotherapy, chemotherapy was initiated, comprising cisplatin, pemetrexed, and atezolizumab. As an antiemetic drug, dexamethasone was administered at 12 mg on day 1 and 6 mg on days 2 and 3.
The patient presented with nausea of Grade 1 on day 1, and there were no remarkable changes in his physical presentation or laboratory data until day 6. On day 7, his body temperature increased to 40°C. The fever was not ameliorated by the administration of acetaminophen and non-steroidal anti-inflammatory agents. He presented with no focal symptoms other than a fever. Blood and urine cultures were negative.
On day 10, a rash appeared on his trunk and limbs. Since the high fever continued, full-body computed tomography was performed on day 13, with no evidence of infectious foci. In the afternoon on day 10, his consciousness deteriorated (Glasgow Coma Scale E2V3M4), and he was unable to maintain a seated position. S-ferritin increased to 5,775 ng/mL, and C-reactive protein (CRP) was 20.91 mg/dL. His serum glucose level was normal. The lymphocyte count decreased to 180 /μL, and the platelet count was 68,000 /μL. The function of the thyroid and adrenal glands was normal.
The clinical presentation, including the fever, confusion, elevated ferritin, thrombocytopenia, and lymphocytopenia, were consistent with systemic immune activation or CRS. For the differential identification of the viral infection, we measured the titer of viral antibodies (EB VCA IgG: 80 times, EB EA: <10 times, EBNA: 2.3 times, and human palbo-virus 19 IgM: 0.30 times). These data did not suggest viral infection as a cause of the patient’s symptoms. There was no increase in the levels of serum uric acid, potassium, calcium, or creatinine; we therefore excluded the possibility of tumor lysis syndrome. High-dose corticosteroid pulse therapy was provided with 1,000 mg of methylprednisolone for 3 days. On day 11, his body temperature remained high, around 39°C; we therefore decided to add 4 mg/kg body weight of tocilizumab according to the strategy for treating CRS (4).
His body temperature decreased on day 12, but hypotension and tachycardia were observed. On day 15, his blood pressure decreased to approximately 60 mmHg. The heart rate was 110 bpm, and SpO2 was 92% with an oxygen supply of 4 L/min. He was in a state of shock; therefore, hemodynamic support with intravenous fluids followed by vasopressor support with dopamine and noradrenaline was initiated. Transthoracic echocardiography revealed diffuse hypokinesis with an ejection fraction of 20% after being within the normal range 5 days before. Maximum doses of dopamine and noradrenaline were provided.
On day 16, his AST and ALT levels increased to 1,468 and 2,537 U/L, respectively. S-ferritin peaked at 56,520 ng/mL, and his renal function worsened (creatinine, 1.44; blood urea nitrogen, 80). X-ray showed congestion, and brain natriuretic peptide increased to 2,259 pg/mL. Electrocardiography revealed no evidence of ischemic change, and the s-creatine kinase level was normal. We therefore considered ischemic heart disease unlikely and he developed acute heart failure - one of the clinical symptoms of CRS.
His blood pressure gradually increased, and catecholamine therapy was tapered. After high-dose steroids pulse therapy, we decreased the steroid dose to 125 mg prednisolone and continued this dose for 7 days. The CRP level steadily decreased after pulse therapy and the addition of tocilizumab. The IL-6 levels increased from 13.1 pg/mL on the day of tocilizumab initiation to 301 pg/mL after 2 days, but the value subsequently decreased (Fig. 1). Regarding the coagulation function, the fibrinogen level decreased to 53 μg/mL, and the fibrin degradation product level increased to 50.8 μg/mL on day 19. He met the diagnosis criteria of disseminated intravascular coagulation (DIC); therefore, we decided to administer fresh-frozen plasma if the DIC state persisted.
Figure 1.
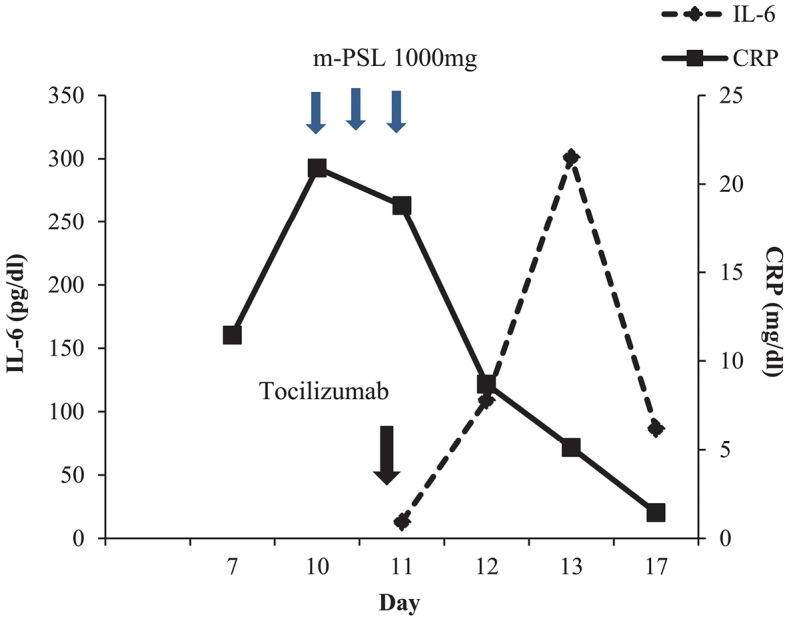
Changes in CRP and IL-6 levels. CRP: C-reactive protein, IL-6: interleukin 6
By day 21, his ejection fraction had recovered to 45%, and dopamine and noradrenaline therapy was discontinued. We decreased the prednisolone dose to 60 mg, with a 5-mg/week tapering regimen. His ejection fraction recovered to 74% on day 25, and the fibrinogen level normalized on day 35. He was discharged from the hospital on day 55. Chemotherapy was re-initiated, including cisplatin and pemetrexed, but no ICIs, and no recurrence of CRS was observed.
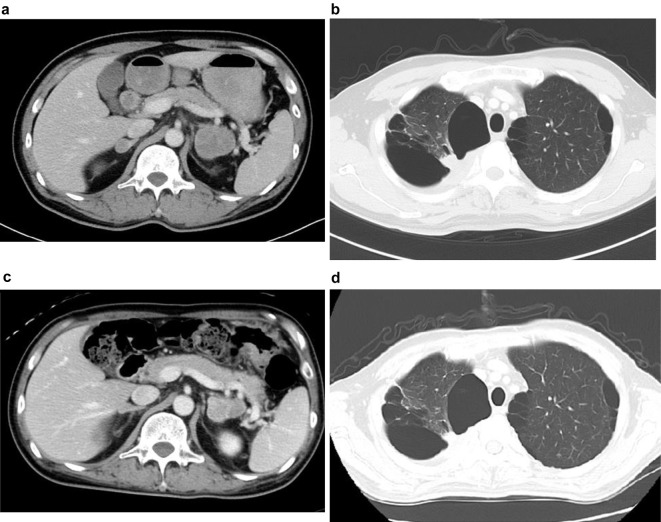
The primary lesion of the lung and adrenal metastasis shrank from 13 and 42 mm before chemotherapy to 10 and 35 mm after the first cycle of chemotherapy, respectively (Fig. 2). He continued to take cisplatin and pemetrexed therapy after discharge, and he ultimately survived for 25 months from the beginning of the first cycle of chemotherapy.
Figure 2.
a: Adrenal metastasis before chemotherapy. b: Primary lesion of the lung before chemotherapy. c: Adrenal metastasis on day 57. d: Primary lesion of the lung on day 57.
Discussion
For patients with metastatic non-small-cell lung cancer, the addition of a programmed death-1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitor to platinum-based doublet chemotherapy has resulted in a significantly higher overall survival and progression-free survival than chemotherapy alone (5,6).
CRS involves various symptoms, beginning with a fever, and may result in severe multi-organ dysfunction due to a systemic inflammatory response (7). Although a relatively common side effect of CAR-T therapy, CRS appears to be a rare side effect of PD-1 or CTLA-4 inhibitor treatment (2,4,7).
To our knowledge, there have only been eight reported cases of CRS induced by ICI therapy, including two cases of lung cancer (8,9). CRS is a non-antigen-specific toxicity; hence, the management of this adverse event may be different from that of other irAE. CRS does not exhibit antigenic toxicity and may develop due to the excessive release of inflammatory cytokines by activation of lymphocytes and macrophages in response to ICI therapy. CRS is more commonly associated with mAb infusions and CAR-T therapy than ICI. The activation of immune cells by CRS may be a common factor seen with ICI and other treatments. The mechanisms underlying the CRS-related cytokine release during ICI treatment are unknown; however, the duration of CRS after treatment is sometimes longer in patients undergoing treatment with ICI than in those undergoing treatment with mAbs and CAR-T. This may be due to the magnitude of immune cell activation, as the T-cell activation after mAbs infusions and CAR-T therapy is stronger than that after ICI therapy.
In the present case, the patient developed a high fever with disturbance of consciousness, acute heart failure, and DIC after chemotherapy containing a PD-L1 inhibitor. The clinical presentation met the criteria of Grade 3 CRS (7). The risk factors of CRS after ICI therapy are unknown, and there are no obvious common features between our case and previously reported cases. In this case, the patients showed high levels of CRP (approximately 7.0 mg/dL) before the initiation of chemotherapy. This suggests that a state of high inflammation might cause CRS, but there is no conclusive evidence to support this notion. The patient recovered after high-dose steroid pulse therapy and the administration of tocilizumab. The recommended first-choice treatment of CAR-T therapy-related CRS is tocilizumab, with corticosteroid therapy being the second choice. However, the pathogenesis of CRS following ICI remains unclear, and no treatment strategy has yet been established. The patient's acute heart failure developed after the decrease in body temperature. Drug-induced heart failure by tocilizumab was unlikely since there were no previous reports on this. He and his family declined to undergo a cardiac catheter test, so we could not test for the existence of coronary artery disease. However, there was no ST-change in the electro-cardiogram and no asynergy of the heart wall during follow-up, so ischemic heart disease was also considered unlikely.
Cardiomyopathy is also considered a clinical signs of CRS (4,7). We therefore suspected that the heart failure had been induced by high-load cytokine release. As reported in one previous case (4), the CRP level decreased immediately after the initiation of tocilizumab, and the IL-6 levels increased for a short while after the initiation of IL-6 receptor blockade before then decreasing. This elevation in IL-6 levels after IL-6 receptor blockade is considered to be due to antibodies that bind to IL-6 receptor, thereby temporarily increasing the serum IL-6 levels. In the present case, however, it took several days of tocilizumab treatment to alleviate the CRS symptoms, such as heart failure and DIC. Patients usually recover within a few hours after the administration of tocilizumab; however, symptoms may not always completely resolve, and the administration of a second dose of tocilizumab or a second immune-suppression agent should be considered (4).
In August 2017, the Food and Drug Administration approved tocilizumab for the treatment of CRS following CAR-T therapy (3). In this approval, the recommended dose of tocilizumab was 8 mg/kg (12 mg/kg for patients weighing <30 kg). The patient described in this report was treated before this recommendation had been made, so the ideal dose of tocilizumab for CRS was considered to be 4-8 mg/kg (4). We used 4 mg/kg of tocilizumab as the initial dose, but this dose might have been insufficient to achieve the immediate improvement of CRS. Our patient ultimately recovered, but we might have used 8 mg/kg of tocilizumab for the first dose or repeated treatment if prompt improvement could not be obtained.
Another potential reason for the transitory deterioration after tocilizumab administration might be the development of macrophage activation syndrome (MAS) (10). MAS is a multi-organ dysfunction due to the release of cytokines, including IL-1, IL-6, IL-18, and tumor necrosis factor-α. It may develop after tocilizumab therapy for autoimmune diseases, such as systemic juvenile idiopathic arthritis, and be characterized by hemophagocytosis, hyperferritinemia, liver dysfunction, and coagulopathy. We did not measure the levels of cytokines other than IL-6 and did not perform bone marrow aspiration; therefore, we cannot conclude whether or not MAS occurred in this case.
CRS is a rare but life-threatening complication. The initial symptoms of CRS, such as a high fever and low-blood pressure, mimic more common complications during chemotherapy combined with ICI therapy, including septic shock and adrenal insufficiency. In addition, the pattern of cytokines released in case of CRS induced by ICI is not fully known, so a harmonized treatment strategy remains to be established. It is important to be aware of CRS as a cause of severe multi-organ dysfunction after the initiation of ICI therapy, including a strategy for the treatment of any imminent cytokine storm.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 15: 47-62, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23: 943-947, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124: 188-195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378: 2288-2301, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078-2092, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 6: 56, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michael L. Adashek a, d Marvin Feldman. Cytokine Release Syndrome Resulting From Anti-Programmed Death-1 Antibody: Raising Awareness Among Community Oncologists. J Oncol Pract 15: 502-505, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Kogure Y, Ishii Y, Oki M. Cytokine release syndrome with pseudoprogression in a patient with advanced non-small cell lung cancer treated with pembrolizumab. J Thorac Oncol 14: e55-e57, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol 12: 259-268, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]