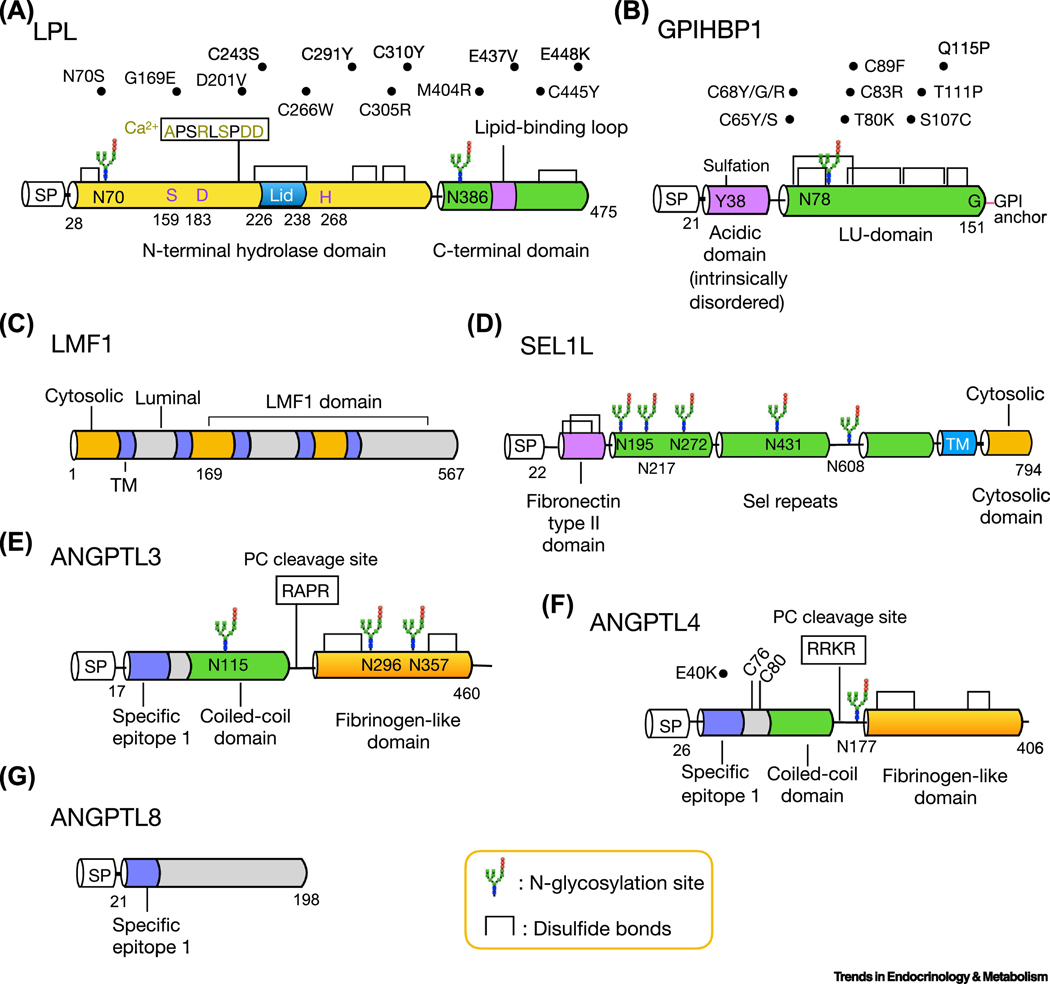
Figure 1. Functional domains, post-translational modifications and disease mutants of LPL and its regulators.
Schematic diagrams of amino acid structure of LPL (a), GPIHBP1 (b), LMF1 (c), SEL1L (d), ANGPTL3 (e), ANGPTL4 (f) and ANGPTL8 (g). The cytosolic, transmembrane and luminal domain of LMF1 are colored with orange, purple and gray, respectively (c). The two cysteine residues of ANGPTL4, C76 and C80, are required for its oligomerization (f). Several human mutations of LPL (a), GPIHBP1 (b) and ANGPTL4 (f) are listed above the structure. SP: signal peptide; TM, transmembrane domain.