Figure 2. Activation of HIF-1 signaling in nickel-exposed cells contributes to EMT.
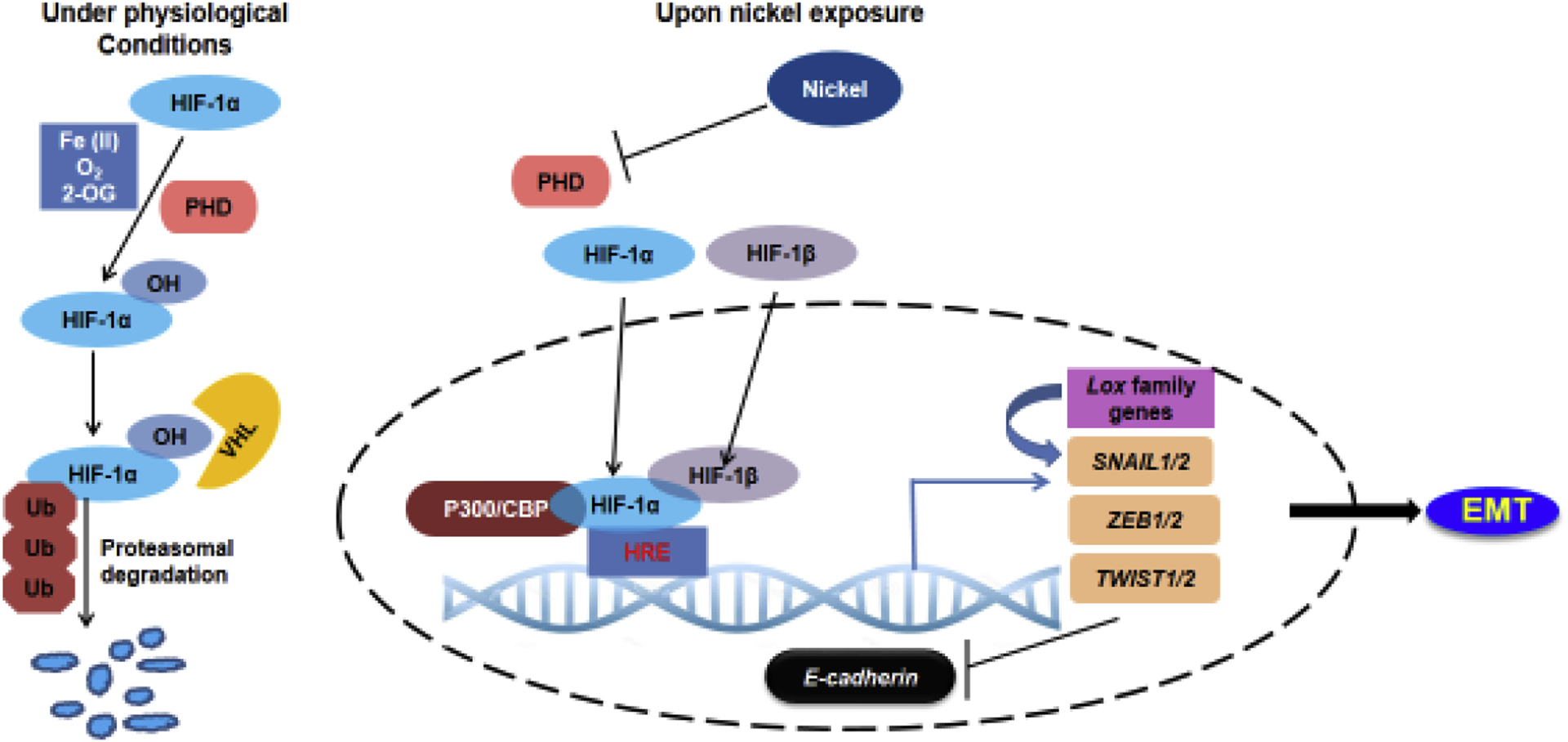
Under physiological conditions, HIF-1α is rapidly degraded via hydroxylation of its oxygen-dependent degradation domain by prolyl-hydroxylases (PHDs). Upon nickel exposure, Ni(II) replaces Fe(II) at the iron-binding site of PHDs causing their inactivation. This leads to the stabilization of HIF-1α, which translocates to the nucleus, dimerizes with HIF-1β and binds the HREs at the promoters of target genes, resulting in their activation. HIF-1 could directly activate EMT master regulators, SNAIL, ZEB and TWIST, by directly binding their promoters. HIF-1 could also upregulate lysyl oxidase genes Lox and LoxL2, which interact with SNAIL and cause E-cadherin repression.
