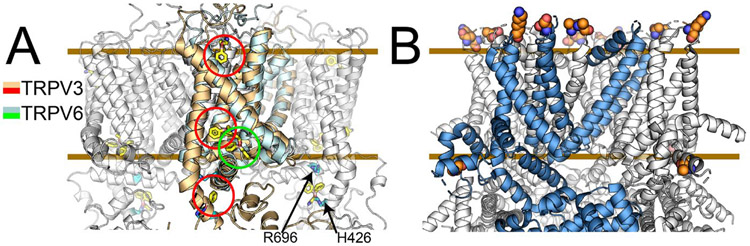
Figure 4. Allosteric coupling in ligand and temperature activation.
Panel A shows structures of 2-APB bound TRPV3 and TRPV6 in gold and cyan respectively. Between the two structural studies, four 2-APB binding sites have been identified in related TRPV channels. These coupled with functional studies suggest that in 2-APB ligand regulation of TRP channels allostery is key to modulating function. Panel B identifies residues essential to exchanging cold-sensing properties between TRPM8 channels from hibernating and non-hibernating rodents. The six residues are far away in space indicating that allosteric networks are key to deciphering these outcomes. Comparing these six mutations with human TRPM8 show that not only are allosteric networks important but also that there must be compensating pairs of residues that impact allostery.