Figure 1.
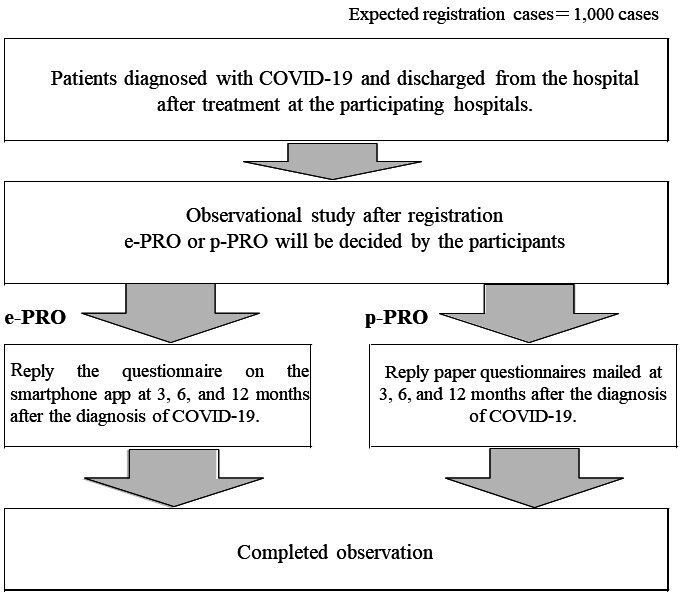
After confirming the research collaborator’s consent to participate and cooperate in this study, basic and clinical information of the research collaborator, such as treatment history, will be registered in the case information registration system, and the electronic patient-reported outcome (e-PRO) or paper patient-reported outcome (p-PRO) will be set up. Participants can choose between e-PRO or p-PRO of their own initiative. The principal investigator will inform the research collaborators of the details of the e-PRO setup by a smartphone app or notification of p-PRO by mail, and collect information regarding sequelae at 3, 6 and 12 months after COVID-19 diagnosis by performing a questionnaire survey.
